Medical Device Product Development Lifecycle
Where are you in the medical device product development lifecycle. Experience the Difference with the MRO Approach Northwood OH Minneapolis MN Irvine CA Sunnyvale CA.

Why Design Controls Matter In Medical Device Development
For devices that incorporate software or for software that are devices in themselves the software shall be developed and manufactured in accordance with the state of the art taking into account the principles of development life cycle risk management including information security verification.

Medical device product development lifecycle. Its either a collection of the actual documents generated during product development or an index of documents and their storage location. Understanding and consideration of the complicated clinical and regulatory requirements early in the product lifecycle could ensure your company gains a competitive by reducing time to market. Regulatory affairs professionals serve a critical function throughout a medical devices product lifecycleleading premarket strategy drafting regulatory submissions and ensuring postmarket compliance.
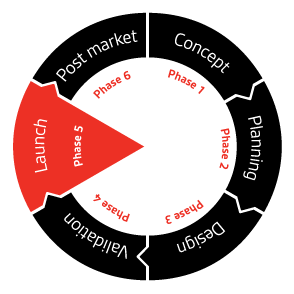
Preclinical clinical regulatory and post-market stages. The concept of a life cycle for medical devices is adopted from the broader idea of a product life cycle PLC. Overview of the different steps of a typical medical device lifecycle from idea to market.
Product development lifecycle consulting is part of a wide range of service offerings that include clinical trial design for medical devices drugdevice combination studies and quality management through our ePremier technology platform. The medical devices regulation MDR and medical device directive MDD require software lifecycle processes. One novel model is to integrate the four key components of development that span the medical products life cycle.
Follow the links below to learn more about how clients gain from our specialized expertise. The 5 Best Tools for Medical Device Product Lifecycle Management 1. These ideation processes generate abstract concepts in need of further development refinement and adaptation to result in a workable base for.
Agile development is an industry disruptor in the noninvasive medical device product lifecycle. 3 Expectations. The FDA defines the design history file in section 82030j.
This course provides a roadmap of the differen. Like all products medical devices begin their lives in a manufacturing plant then sold to the end user and may be used until the natural end of their life cycle. 2 To reduce risk strict single-use regulations around many invasive medical devices along with mandatory legislation in the United States and Europe on the use of products for needlestick injury prevention.
An important distinction and critical element of any design control that defines a product life cycle PLC is that it can play a critical role during a medical device audit or for demonstrating design-control compliance. In the competitive medical device market place ensuring that product development meets all regulatory requirements is essential. MasterControl is a widely-known option for enterprise quality management software eQMS.
2 Thinking Globally During Medical Device Development. The Four Phases of Medical Device Product Development Lifecycle 1. A quality-driven approach can accelerate product development and market access for medical device startups.
Medical device development lifecycle 1. While noninvasive medical device creation is a bit less regulatorily restrictive than devices meant for use inside the human body the process is still a. Download Our Free Guide to Breaking into Regulatory Affairs.
This process of creating testing improving and creating again proves concepts that support designs to guide and educate the next generation of medical professionals. Developing a PLC in tandem with a solid product development process PDP provides the necessary cornerstones for complete design-control and device-management compliance. Accelerating Products to International Markets Tim Blair.
This integrated approach is helping to simplify product development which when done with a single research partner can. Similarly the European Centre for Disease Prevention and Control Healthcare Acquired Infection HAI reports that rates run at between 5 and 8 of patients in most developed countries. Heres a closer look at each of those phases.
Protecting connected medical devices from evolving cyber related threats requires a continuous lifecycle approach whereby cybersecurity is integrated within the product development lifecycle and both complements and re-enforces the safety risk management processes therein. The DHF is a formal document prepared for each medical device that contains all of the required documents from the earlier stages. This phase contains several steps from idea conception the use context of the medical device initial concept development up to proof of concept.
IBM Engineering Lifecycle Management ELM gives medical device manufacturers the traceability and transparency they need to optimize their end-to-end development processes allowing them to be competitive in the market.

Why Design Controls Matter In Medical Device Development

Medical Device Product Life Cycle Page 1 Line 17qq Com
Medical Device Product Development Lifecycle Bsi

Medical Device Software Iec 62304