Fda Class 1 Medical Device Definition
Within or on the body of man or other. Under current law Class I devices are defined as those for which general controls are sufficient to provide reasonable assurance of the safety and effectiveness of the device11 Many Class I devices are exempt from the pre-market notification andor the quality system QS regulation.
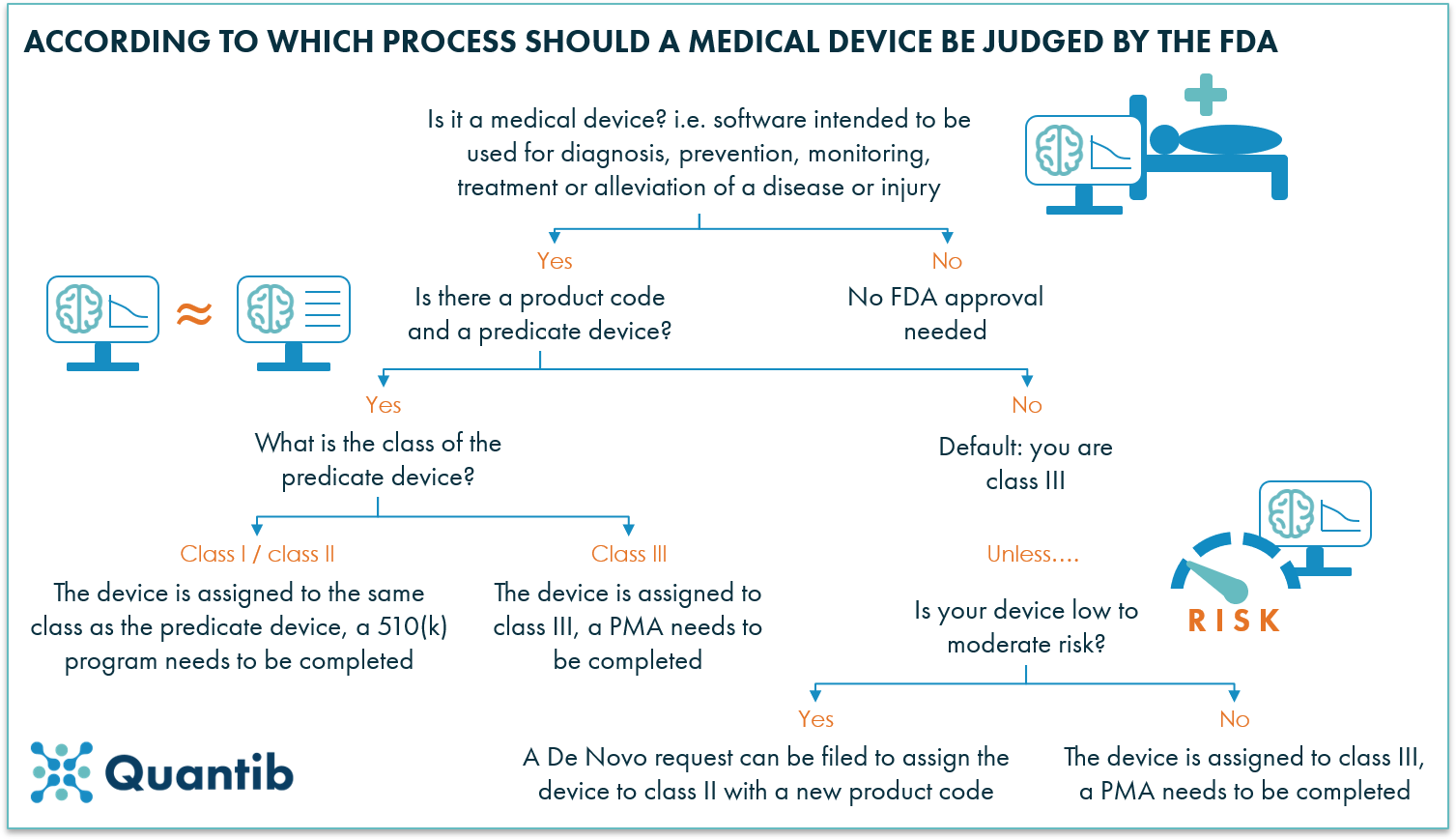
A 101 Guide To The Fda Regulatory Process For Ai Radiology Software
A medical device is defined by law in the section 201 h of the Federal Food Drug and Cosmetic FDC Act.

Fda class 1 medical device definition. Class 1m Measuring Devices. The term preamendments device refers to a device legally marketed in the US. For a medical product also to meet the more restrictive device definition under section 201h of the FDC Act it must i be an instrument apparatus implement machine contrivance implant.
ICDs may contain electrical wire connections which may not be completely insulated. Class I devices have the least regulatory requirements. Class I devices are considered to be at the lowest level of risk of all medical devices and are therefore required to comply with the lowest level of regulatory control.
Class I devices generally pose the lowest risk. The three classes are. A limb orthosis brace is a device intended for medical purposes that is worn on the upper or lower extremities to support to correct or to prevent deformities or to.
8 A device intended for export from the United States. The FDA generally classifies medical devices based on the risks associated with the device and by evaluating the amount of regulation that provides a reasonable assurance of the devices safety and effectiveness. Medical devices are categorized into one of three classes I II or III based on the.
Class II devices are simple devices though they are more complicated than Class I devices. Class 1 Device Recall Ellipse ICD. The design control requirements of Section 82030 of the regulation apply to the design of Class II and III medical devices and a select group of Class I devices.
In this case of devices placed on the market in sterile condition the Notified Body involvement is limited to the aspects of manufacture concerned with securing and maintaining sterile condition. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and effectiveness of the device. The potential patient impact could be.
The regulation is very flexible. Class I and Class II devices specifically exempted by the FDA. Medical device class MDC is a regulatory category defined by the United States Food and Drug Administration FDA.
Class 1s Medical Device Sterile Medical Devices Class 1s medical devices have lowmedium risks perceived. In the United States the FDA has the authority to regulate medical devices before and after they reach the marketplace. Examples of Class I devices include.
Electrical failures were identified in cardioverter defibrillators ICDs due to damaged aluminum wires. Definition of a Medical Device Continued And does not achieve its primary intended purposes through. 7 A veterinary medical device not intended for use in the diagnosis of disease or other conditions in man in the cure mitigation treatment or prevention of disease in man or intended to affect the structure or any function of the body of man.
They are also considered to. Elastic bandages dental floss and enemas.

Fda Medical Device Classification Medical Device Validationpresentationeze

Software As Medical Device Samd Classification And Definitions

Does An Fda Class 1 Medical Device List Exist
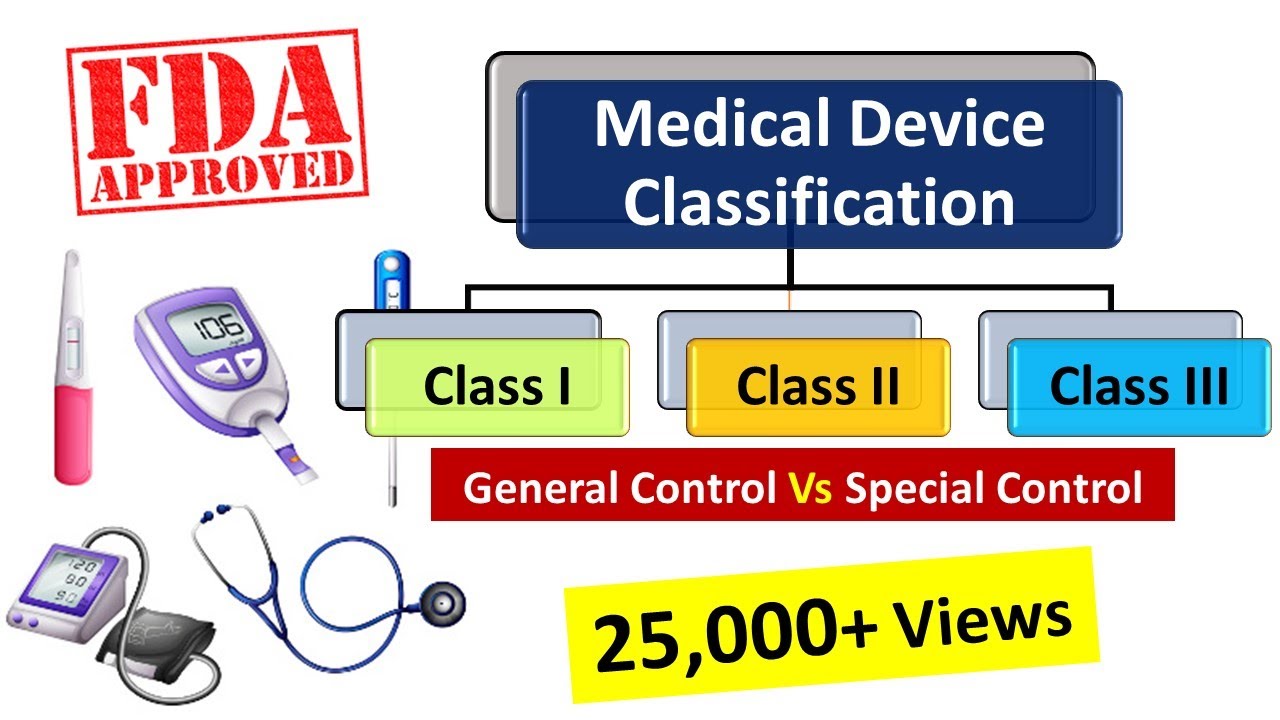
Medical Devices Classification As Per Fda Medical Device Regulations Medicaldevices Fda Youtube