Definition Of A Medical Device Fda
An instrument apparatus implement machine contrivance implant in vitro. Section 201h of the Food Drug Cosmetic Act.
Medical Device Complaint Handling Understanding The Basics Oriel Stat A Matrix Blog
In August 2017 the FDA began a new effort to classify a list of medical device accessories that the Agency believes are suitable for distinct classification into class I.

Definition of a medical device fda. Store at 15-25C 59-77F. The FDA defines a medical device as. The electronic transfer of medical device data.
Lets start with a comparison of the definition of a medical device by FDA and the EU. 1 A device identifier - a mandatory fixed portion of a UDI that identifies the specific version or model of a device and the labeler of that device. An instrument apparatus implement machine contrivance implant in vitro reagent or other similar or related article including a.
Design Verification and validation are essential concepts for the design process of a medical device and it is of fundamental importance to fully understand the differences and the requirements associated with these activities. Certain Key Provisions of the Definition of Device Conceptually all FDA-regulated medical products meet the definition of drug under section 201 g of the FDC Act due to the broader scope. The Federal Food Drug and Cosmetic Act FFDCA is the law under which the FDA takes action against regulated products.
FDC Act defines a device as. In this post we provide an overview of the FDA requirements for design verification and validation. I Hospital means a distinct entity that operates for the.
Store at 2-8C 36-46F. Medical Device definition from the Medical Device Regulation MDR 2017745 The full EU MDR Medical Device definition is. Store between 8-15C 45-59F.
In fact the distinction between the two terms has long been the subject of debate and interpretation. On August 18 2017 section 513 f of the Federal Food Drug and Cosmetic Act FDC Act was amended by the FDA Reauthorization Act of. D Device user facility means a hospital ambulatory surgical facility nursing home outpatient diagnostic facility or outpatient treatment facility as defined in this section which is not a.
An instrument apparatus implement machine contrivance implant in vitro reagent or other similar or related. For heat sensitive products that must not be frozen. H Five-day report means a medical device report that must be submitted by a manufacturer to us under 80353 within 5 work days.
There are also some definitions in the WHO Guidance. Based on the FFDCA the FDA has created the CFR -. Transported within a cold chain and stored at -20C 4F.
According to FDA definition a device is. Definition of a Medical Device. Die FDA definierte Medical Device Data Systems MDDS wie folgt A medical device data system MDDS is a device that is intended to provide one or more of the following uses without controlling or altering the functions or parameters of any connected medical devices.
Medical device means any instrument apparatus appliance software implant reagent material or other article intended by the manufacturer to be used alone or in combination for human beings for one or more of the following specific medical purposes. Safety of medical devices and in vitro diagnostic products in the US. And 2 A production identifier - a conditional variable portion of a UDI that identifies one or more of the following when included on the label of the device.
FDA Updates Definition of Custom Medical Devices 30 October 2016 admin Earlier this month the US FDA updated its definition of a custom medical to include new statutory requirements for custom devices under the Federal Food Drug and Cosmetic Act the FDC Act as amended by the Food and Drug Administration Safety and Innovation Act FDASIA. Intended to affect the. Intended for use in the diagnosis of disease or other conditions or in the cure mitigation treatment or prevention.
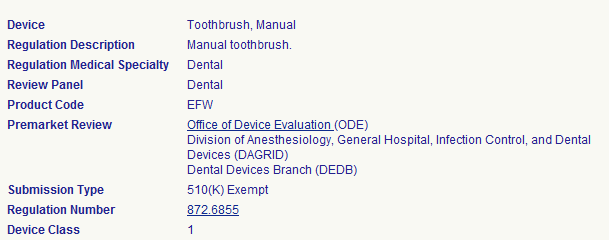
Is regulated by the FDA.
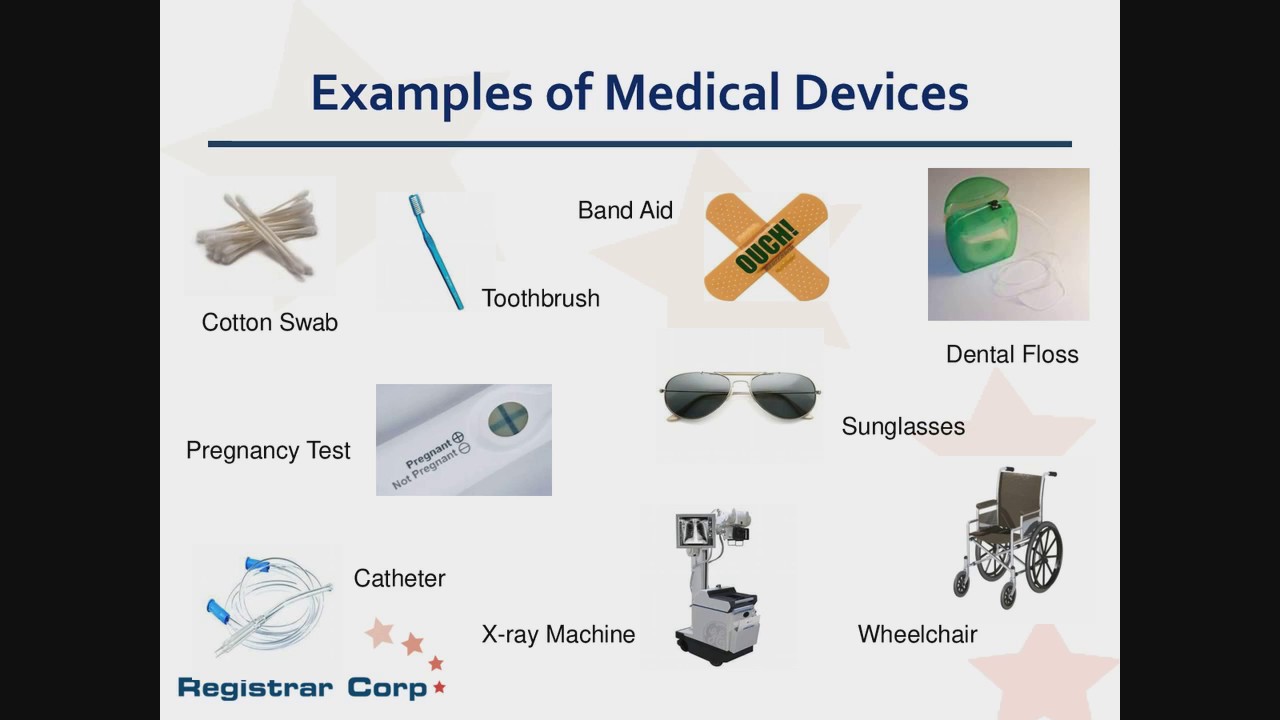
Fda 101 For Medical Devices Youtube
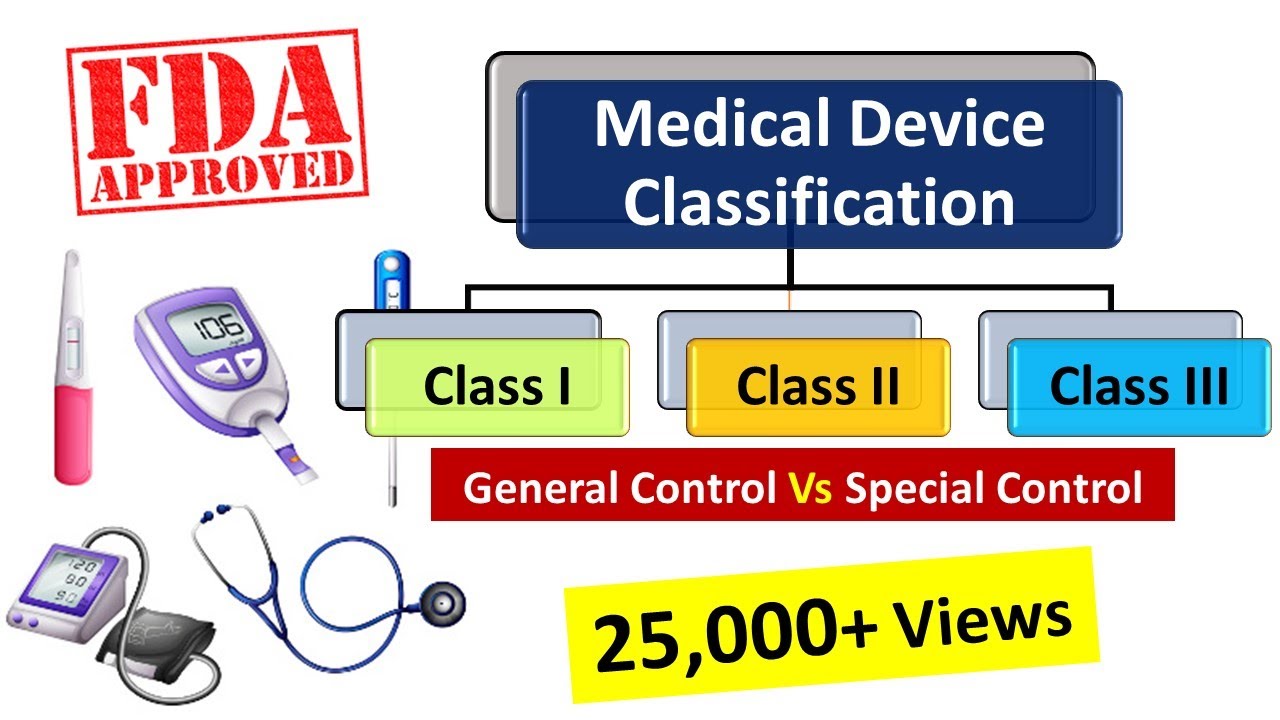
Medical Devices Classification As Per Fda Medical Device Regulations Medicaldevices Fda Youtube

Are You Making A Medical Device Voler Systems