Fda Private Label Medical Device
B The requirement for declaration of the name of the. Medical device labeling is not just the label on your device.
Private Labeled Devices With Fda Approval Medical Device Academy Medical Device Academy
It appears the OEM medical device is imported into the US.
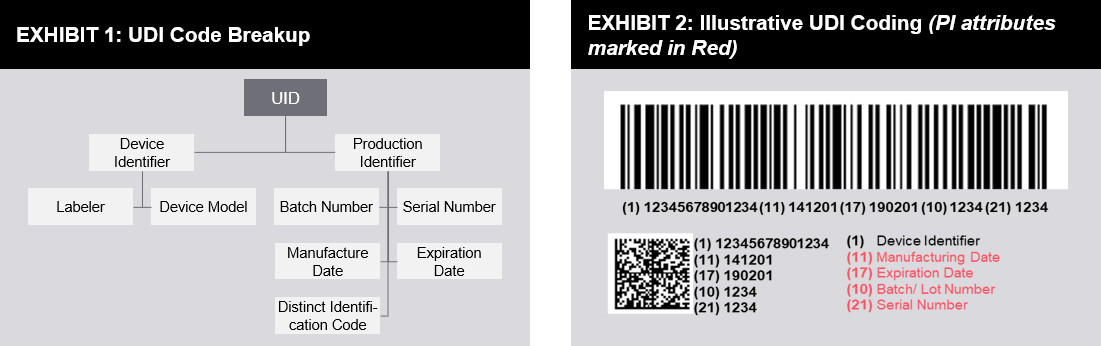
Fda private label medical device. A new UDI representing the private labeler would. 80135 - Voluntary labeling of a device with a unique device identifier. The general labeling requirements for medical devices are contained in 21 CFR Part 801.
A firm that does not participate in the manufacture or processing of a drug but instead markets and distributes under its own trade name and labels a drug product made by someone else is referred to as a Private Label Distributor or PLD. When we talk about labeling were talking about the label s on the pouch on. Private-label goods are available in a wide range of.
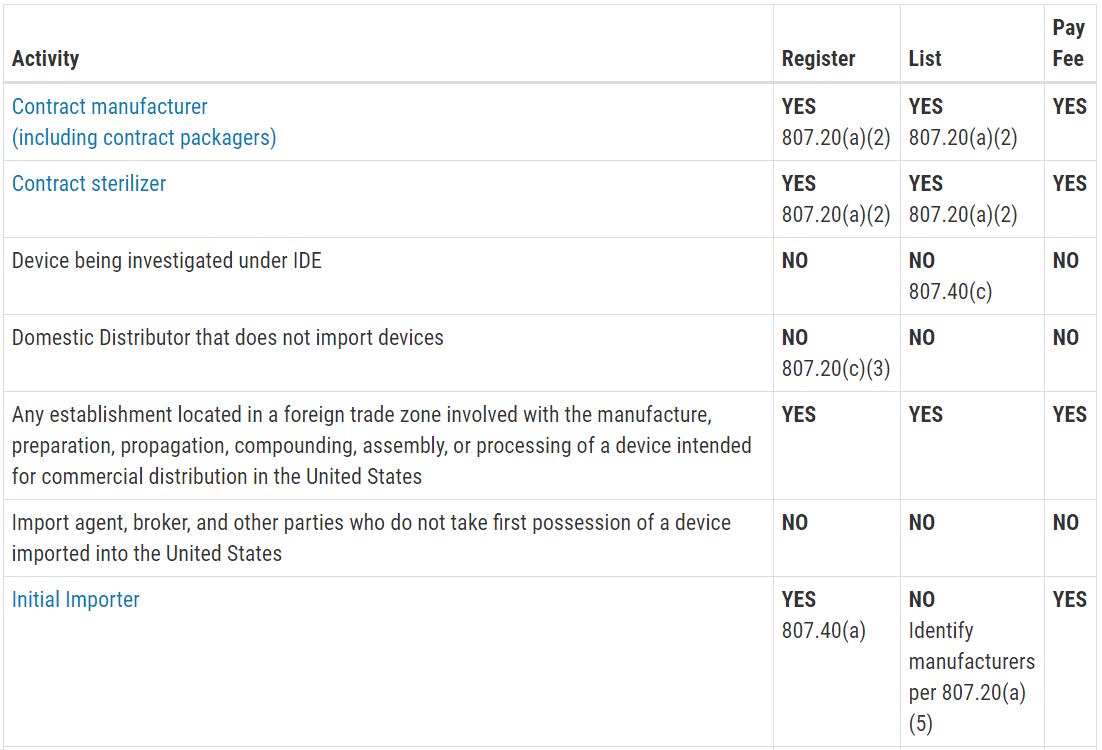
PLDs do not have a registration or listing obligation for drugs they do not manufacture or process. These provisions include having systems in place to handle complaints and to manage mandatory problem reporting and recalls. Therefore there are no fees associated with the examination of private label medical device applications or private label licence amendment.
The FDA requirements for labelling of medical devices are embedded with Quality System Regulation requirements mentioned in 21 CFR Part 820. Private label medical devices are currently exempt from Division 2 - Fees for the Examination of Medical Devices Licence Applications contained in Part 3 - Medical Devices Fees of the Fees in Respect of Drugs and Medical Devices Regulations. Of the fourteen laws currently administered by FDA three directly address the labeling of medical devices.
FDA to clarify role of off-label uses in medical device approvals September 23 2020 By Nancy Crotti The FDA has released proposed regulations to make clear that off-label use of a device alone will not be enough to sway the agency to give its blessing to that use. Whereby the Original Equipment Manufacturer OEM is the party from which the product is bought and the private. In the medical-device world previously certified products are often being bought by companies with the intention to bring them onto the European market under their own trade name.
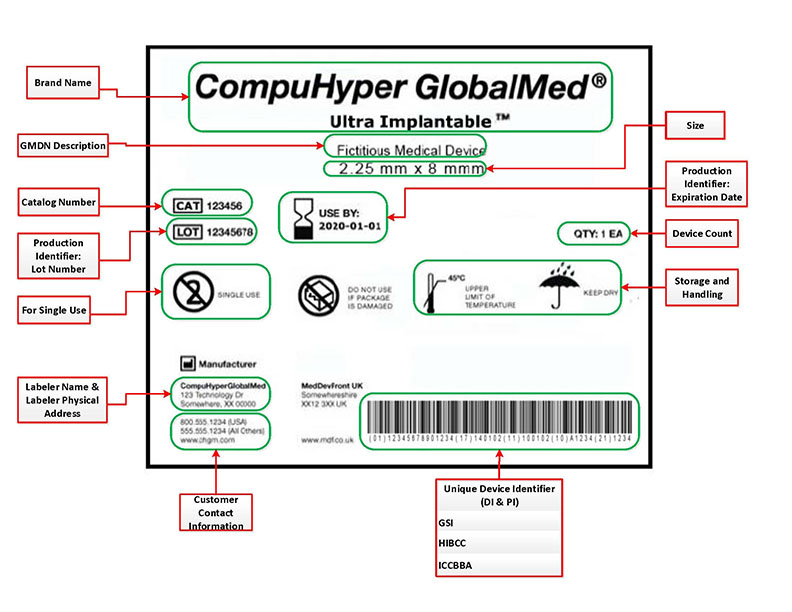
80130 - General exceptions from the requirement for the label of a device to bear a unique device identifier. A The label of a device in package form shall specify conspicuously the name and place of business of the manufacturer packer or distributor. O The Food Drug and Cosmetic FDC Act - The FDC Act applies to food drugs.
Name and place of business of manufacturer packer or distributor. As mentioned above the private labeler is considered a relabeler by the FDA and as such becomes the UDI Labeler company responsible for placing UDI on the product and for reporting UDI data to the FDA Global UDI Database GUDID. Medical device labeling is just the label on the device.
And then relabeled with the private labelers name. Later sections in this chapter discuss. Do we need to include the proprietary names for each private-label device.
Private Label for Medical Device Manufacturers Private-label products or so called private brands are products that are manufactured or provided by one company for offer under another companys brand name. A The label of a device in package form shall specify conspicuously the name and place of business. Name and place of business of manufacturer packer or distributor.
This is commonly known as bringing a product onto the market under private label or plainly private labeling. The private label manufacturer must notify Health Canada. Yes your device listings should include all of the current proprietary names that are used to market the devices you.
All other provisions of the Food and Drugs Act and Medical Devices Regulations apply to a private label medical device and private label manufacturer and are the responsibility of the private label manufacturer. This includes activities for labelling control incoming control of materials including labelling and design documentation to ensure labels have adequate space on the device and packaging. These regulations specify the minimum requirements for all devices.
Before we go any further we have to get one major misconception cleared up. CFR - Code of Federal Regulations Title 21.

Unique Device Identification Udi For Medical Devices Futurebridge

Private Labeled Devices With Fda Approval Medical Device Academy Medical Device Academy

Unique Device Identification Udi For Medical Devices Futurebridge