Fda Performance Standards Medical Devices
After FDA has decided to recognize a standard we will update our online database to reflect the decision even before formal recognition of the standard occurs by publication in the Federal. Medical device companies should be encouraged to consider inclusion in the program early in device development to take full advantage of a fast-track approval.

The Regulation Of Wearable Medical Devices Trends In Biotechnology
102 Zeilen Medical electrical equipment Part 2-31.
Fda performance standards medical devices. New 2020 lists of harmonised standards for medical devices are now available. Recall from your previous courses in BIOE that medical devices as defined by the FDA can range from simple tongue depressors to complex programmable pacemakers with microchip technology and laser surgical devices. Compliance with the Essential Principles of.
Specific Performance Standards for Electronic Products 21 CFR 1020 - 1050 Television receivers 21 CFR 102010 Cold-cathode gas discharge tubes 21 CFR 102020 Diagnostic x-ray systems and. They must establish and follow quality systems to help ensure that their products consistently meet applicable requirements and specifications. Guidance on Electrosurgical Devices and the Application of the Performance Standard for Electrode Lead Wires and Patient Cables Issued November 1999.
FDA issued guidance Thursday with its recommendations for information about non-clinical bench performance testing for medical devices that manufacturers should include in premarket submissions. Modification to existing standards include anesthesiology equipment biocompatibility of devices safety and performance standards for cardiovascular stents and non-invasive automated. Provides definition of a medical device FDA classification panels conducted initial classification of preamendments medical devices ie Class I II III.
Particular requirements for the basic safety and. F Performance requirements - 1 Protective housing. Must comply with regulations set out by the FDAs Center for Devices and Radiological Health CDRH.
Guidance for Industry FDA ReviewersStaff and Compliance. The Food and Drug Administration FDA developed this document to provide guidance to industry and FDA staff about the appropriate use of national and international voluntary consensus standards. Nefab is ready for this with our global coverage of ISTA certified test labs and our engineers can apply the right packaging design choices.
ISTA 3 packaging and transport standards are now official standards to be used for FDA approval on medical devices for the US market. The guidance outlines what to include in test report summaries test protocols and complete test reports. Each laser product shall have a protective housing that prevents human access during operation to laser and collateral radiation that exceed.
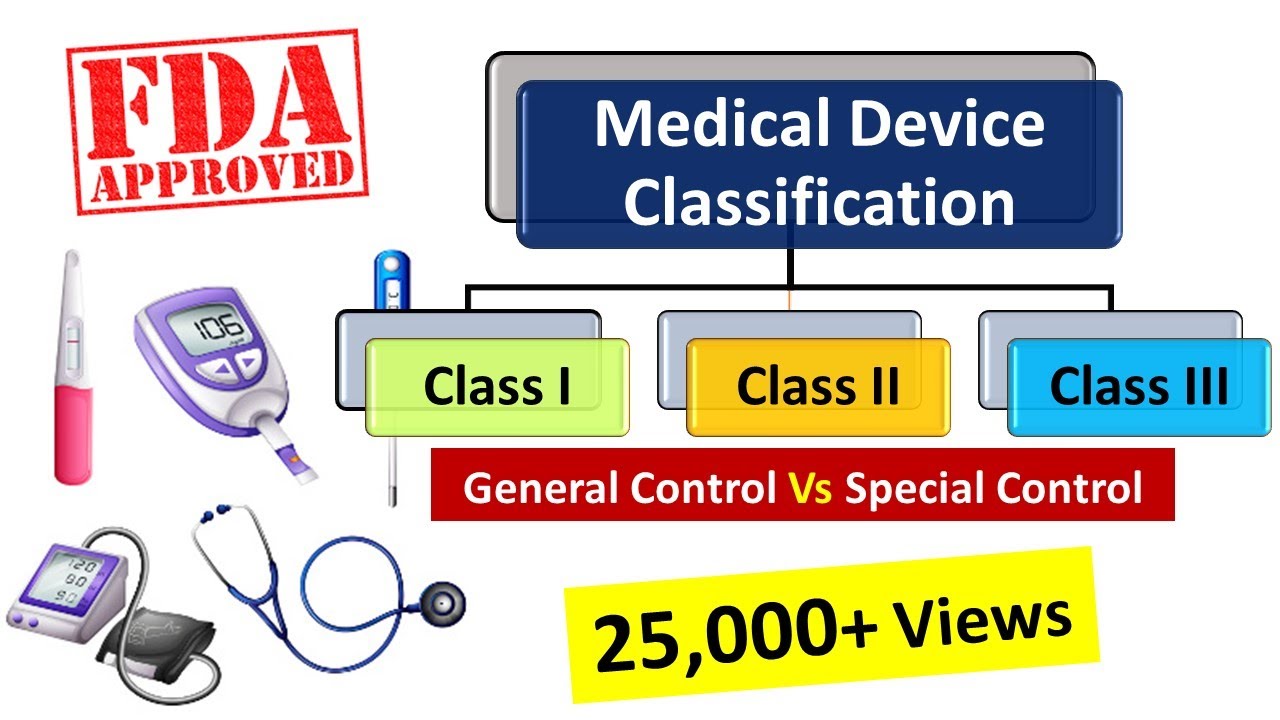
As which standards are applicable. Medical device and IVD medical device is safe and performs as intended by the manufacturer. Class I devices pose the least risk.
For Industry and FDA Staff. The post FDA Introduces the Safer Technologies Program for Medical Devices appeared first on MedTech Intelligence. Manufacturers of medical devices distributed in the US.
B The Food and Drug Administration may determine that a performance standard as described under special controls for class II devices in 8607 b of this chapter is necessary to provide. The FDA has three classes for medical devices Class I Class II and Class III based on the level of risk the device poses to the user. Guidance Document for Powered Muscle Stimulator 510 ks Issued June 1999.
The new lists of references of harmonised standards for medical devices have been published OJ L 0901 of 25 May 2020. Essential principles of safety and performance provide broad high-level criteria for design production and postproduction throughout the life-cycle of all medical devices and IVD medical devices ensuring their safety and performance. They can be found below.

Medical Devices Classification As Per Fda Medical Device Regulations Medicaldevices Fda Youtube

Medical Device Software Iec 62304

Device Regulations The New Medical Device Regulation The Applicability Of Article 117 To Medicinal Products
