Fda Definition Intended Use Medical Device
Its purpose is not to describe what the device is intended to be used for. FDC Act defines a device as.

Market Access For Medical Software In The United States Vde Medical Devices And Software
Intended use and defines appropriate specifications.

Fda definition intended use medical device. Definition of a Medical Device. A lot of people think that intended use means what your medical device could be used for. Instead it should lay out the claims of what your device is meant to do.
Use design controls to build quality safety effectiveness and savings into your medical device. An instrument apparatus implement machine contrivance implant in vitro. And once again thats not what intended use means intended use is all about what we say our label claims.
Medical Device Accessories FDA Skip to main content. Medical devices include in vitro diagnostic products such as general purpose lab equipment reagents and test kits. Definition of the term intended use of medical devices The term intended use includes medical devices strictly speaking in two aspects.
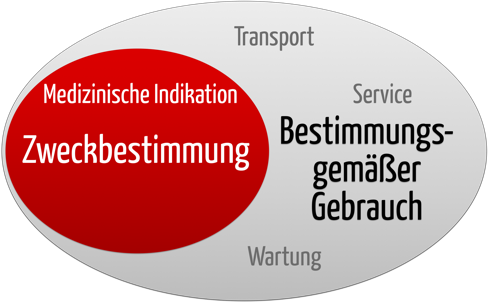
The intended use of a device is critical for determining its classification and is usually defined during the initial regulatory stages. NORMAL USE should not be confused with INTENDED USE. The FDA oversees their safety and effectiveness just like contact lenses that correct your vision.
While both include the concept of use as intended by the MANUFACTURER INTENDED USE focuses on the medical purpose while NORMAL USE incorporates not only the medical. 8014 Meaning of intended uses. These generally are simple statements of no more than 2-3 sentences that outline the devices tools specific use within a particular market.
Examples include diagnostic ultrasound products x-ray machines and medical lasers. So its all about what we say and Ive got a lot of examples we can use but intended use is first and foremost about what we say our device is to. The words intended uses or words of similar import in 8015 801119 and 801122 refer to the objective intent of the persons legally responsible for the.
In the regulatory world its what I call the high level labeling. An accessory is a finished device that is intended to support supplement andor augment the performance of one or more parent devices. This is more of a trend among assessors rather than a regulatory requirement.
Certain electronic radiation emitting products with medical application and claims meet the definition of medical device. Section 201h of the Food Drug Cosmetic Act. Intended Use and the 510k FDA wants you to be very to the point about your stated intended use on your 510k submissions.
Intended Use is defined as the general use statement of a medical product or what is the use of the device1 It tells the user what the device is meant to do over the lifetime of the product. L Manufacturer means any person who manufactures prepares propagates compounds assembles or processes a device by chemical physical biological or other procedure. Indications of use the conditions or reasons for using the device.
However the intended use is often misunderstood. The intended performance of a device refers to the intended use for which the device is labeled or marketed as defined in 8014 of this chapter. B Commercial distribution means any distribution of a device intended for human use which is held or offered for sale but does not include.
The authorised use including the intended use but also other planned actions of the user with this medical device such as storage transport update or cleaning. Decorative contact lenses are considered medical devices. The actual medical purpose that is which disease or injury you can diagnose treat or monitor.
Operation including routine inspection and adjustments by any USER and stand-by according to the instructions for use or in accordance with generally accepted practice for those MEDICAL DEVICES provided without instructions for use Note 1 to entry. Intended use what you say on the label that the device is to be used for. However it can be burdensome on companies.
A Act means the Federal Food Drug and Cosmetic Act.

Software As Medical Device Samd Classification And Definitions

Fda S Breakthrough Devices Program What You Should Know

Market Access For Medical Software In The United States Vde Medical Devices And Software