Fda Class 1 Medical Device List
In order to import your listed device into the United States youll need to provide the registration number or the owneroperation number as well as the listing number of the device. 2018-0002 stipulate that a guidance document containing the list of medical devices per classification shall be issued by the Food and Drug Administration FDA Center for Device Regulation Radiation Health and Research CDRRHR.
List Of Medical Devices By Product Code That Fda
Initial list of medical devices with risk classification was issued through FDA Circular No.

Fda class 1 medical device list. For Class 1s 1m 1r medical devices inspection is limited to sterilitymetrologyre-use as applicable the conformity assessment route is as follows. General limitations to the exemptions. However these manufacturers are required to register their establishment and list the generic category or classification name.
Devices on the class I exemption list include enzyme controls tonometers parallelometers irrigating dental syringes finger cots and protective restraints for. Technical Documentation Technical File preparation as per Annex II and III. If you have a generic Class II medical device you can discover whether it is exempt from a 510k filing by searching the FDA Product Classification database.
The US Food and Drug Administration has published a finalized list of accessories to be designated as low-risk Class I medical devices in accordance with the FDA Reauthorization Act of 2017 FDARA. 2020-001 entitled Initial. ICDs may contain electrical wire connections which may not be completely insulated.
Electrodes for EEG or ECG. As per rule 1 Class 1 is the medical devices either do not touch the body part or just touch the intact skin. A listing of Class I and Class II devices exempt from 510 k requirements is available on the Medical Device Exemptions 510 k and GMP Requirements website.
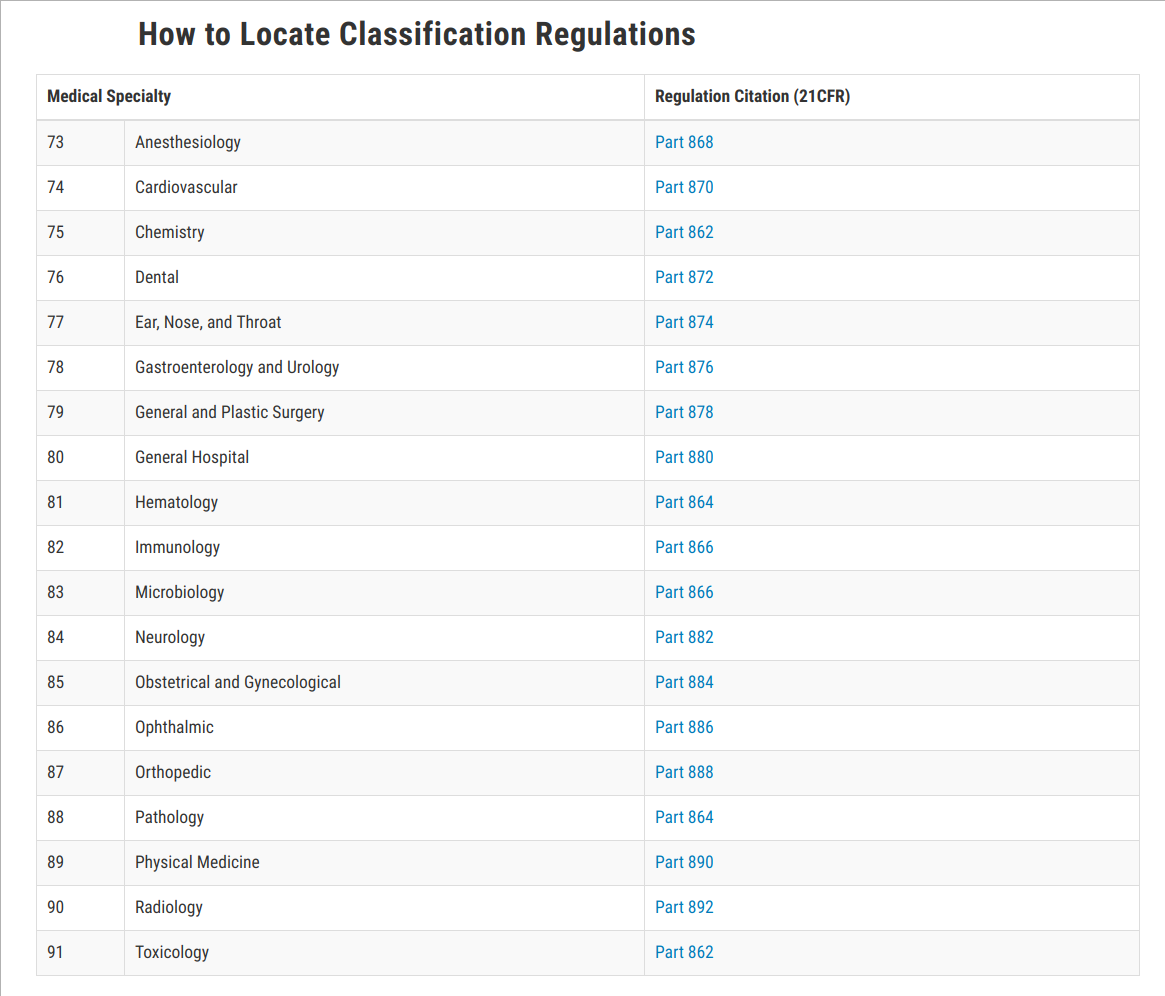
If a manufacturers device falls into a generic category of exempted class I devices as defined in 21 CFR Parts 862-892 a premarket notification application and FDA clearance is not required before marketing the device in the US. Device Class and Regulatory Controls. Class I General Controls.
Class I medical devices are those products deemed to be low-risk and as such are subject to the least amount of regulatory control. Subsections V1 and V2 of AO No. Releasable establishment registration and listing information under the Freedom of Information Act is available by.
The FDA released an exemption list in early 2018 which exempts over 800 generic Class I and II medical devices from the 510k process. Corrective glasses and frames. Declaration of Conformity as per Annex IV.
If a manufacturers device falls into a generic category of exempted class I devices as defined in 21 CFR Parts 862-892 a premarket notification application and FDA clearance is not required. Affixing the CE mark as per Annex V. Establishment Registration and Medical Device Listing Files for Download.
List of Medical Devices by Product Code that FDA classifies as Implantable Life-Saving and Life-Sustaining Devices for purposes of Section 614 of FDASIA. External patient support devices such as hospital beds patient hoists walking aids wheelchairs stretchers. Additional accessories may be identified for low-risk classification by 2024.
Electrical failures were identified in cardioverter defibrillators ICDs due to damaged aluminum wires. However you are required to register your establishment with the FDA by registering your company and listing the medical devices using the FDA. Class II General Controls and Special Controls.
Medical devices are classified in one of three groups depending on the amount of risk associated with their use. The potential patient impact could be. FDAs final rule will go into effect May 13 2019.
Registration and listing information is submitted by using FDAs Unified Registration and Listing System FURLS Device. If your device falls into a generic category of exempted Class 1 devices as defined in 21 CFR Parts 862-892 a Premarket Notification 510k clearance may not be required unless you are introducing a new and unique technology or unless you modifychangeexceed the intended use. Class 1 Device Recall Ellipse ICD.

Does An Fda Class 1 Medical Device List Exist
Does An Fda Class 1 Medical Device List Exist

Medical Devices Angela N Johnson
Medical Devices Biomedical Engineering Libguides At City College Libraries
