Fda Adverse Event Definition Medical Device
An adverse event occurring from abuse of the product. The openFDA device adverse event API returns data from Manufacturer and User Facility Device Experience MAUDE an FDA dataset that contains medical device adverse event reports submitted by.
The following definitions of terms apply to this section.
Fda adverse event definition medical device. Manufacturer and User Facility Device Experience MAUDE data MAUDE data contain reports received by the FDA of adverse events involving medical devices. Any adverse event associated with the use of a biological product in humans whether or not considered product related including the following. For more information regarding adverse event reporting for authorized medical devices please refer to Section IIIE2 of the FDA guidance document Emergency Use Authorization of Medical Products.
Mandatory reporters that is manufacturers device user facilities and importers are required to submit certain types of reports for adverse events and product problems to the FDA about medical. For the most up-to-date version of CFR Title 21 go to the Electronic Code of Federal Regulations eCFR. An adverse event occurring in the course of the use of a biological product in professional practice.
An adverse event occurring from overdose of the product whether accidental or intentional. The objective of the MDR regulation is to provide a mechanism for FDA and manufacturers to identify and monitor significant adverse events involving medical devices so that problems may be detected. Postmarketing Adverse Event Reporting for Medical Products and Dietary Supplements During an Influenza Pandemic OCETCDERCBERCFSANCDRH February 2012.
The MDR reports for adverse events that involve the devices that Firm A manufactured. So adverse events are non-device-related non-serious medical occurrences. Notice that Notice that the word event is used for untoward medical occurrences not related to the investigational.
Guidance for Industry. The information on this page is current as of April 1 2020. Adverse event means any untoward medical occurrence associated with the use of a drug in humans whether or not considered drug.
C Caused or contributed means that a death or serious injury was or may have been attributed to a medical device or that a medical device was or may have been a factor in a death or. 57 adverse event means any untoward medical occurrence unintended disease or injury or any untoward clinical signs including an abnormal laboratory finding in subjects users or other persons in the context of a clinical investigation whether or not related to the investigational device. If you are a device user facility you must report deaths and serious injuries that a device has or may have caused or contributed to establish and maintain adverse event files and submit summary.
Http Www Fda Gov Files About 20fda Published Fda Medwatch Adverse Event Reporting Case Study Pdf

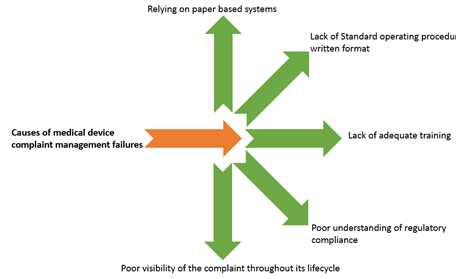
Complaint Management And Medical Device Reporting Overview
Http Www Fda Gov Files About 20fda Published Fda Medwatch Adverse Event Reporting Case Study Pdf

