Us Agent Fda Medical Device
US FDA removes some software functions from medical device classification regulations Apr 20 2021 The US Food and Drug Administration FDA published a ruling in the Federal Register on April 19 adjusting the identifications of medical software in classification regulations to exclude functions that no longer fall under the scope of the agencys regulatory authority. 2 register their facility with the FDA prior to exporting devices for import into the US.

Us Fda Agent Services For Foreign Companies Fda Us Agent Representation 5 Star Ratings
Your US Agent serves as a liaison between your company and the FDA.

Us agent fda medical device. Emergo can act as your Official Correspondent and US Agent. The FDA US agent must either reside in the US. The manufacturer is not registered with FDA or not appointed a US Agent.
Or maintain a business in the US. Are required to register with the US FDA and have an FDA US agent. The common reasons FDA detain a medical device.
When applicable the database also. Emergo provides US Agent services to more than 300 medical device companies so we know how to handle correspondence with the FDA. Pragmatic offer services to act US Agent for Medical Device companies.
The medical device is not listed with FDA or doesnt have 510 K clearance or PMA. Responsibilities of US Agent The US agent acts to communicate between. Establishments located outside of the United States which are involved in the production and distribution of medical devices for commercial purposes are required to.
All the foreign medical device companies who are required to comply with FDA registration requirements should appoint US Agent for communication with FDA. The agent must be able to communicate with FDA during normal business hours for routine. You may search the medical device registration and listing database for registration information for any medical device firm that is registered with the FDA.
It is mandatory all foreign food drug medical device manufacturers and exporters applying for FDA Facility Registration Establishment Registration to appoint a US FDA Agent. We are one among the most reputed US FDA Agent to assists food drugs medical devices nutraceutical and cosmetic manufacturers and exporters across the Globe. GeBiao is a leading Medical Device and IVD compliance consulting firm providing valued product regulatory compliance services and tailored solutions to help our clients gain competitive advantage by reducing business risks.
Medical device companies manufacturers importers exporters etc must register list their devices and foreign companies must designate a US Agent FDA Agents. US Agent for Medical Device companies. It is mandatory to designate FDA US Agent by establishments located outside USA.
The US FDA Agent must be a resident of the United States or maintain a physical place of business in the US. When you appoint us as. Provides FDA medical devices establishment registration and medical device Listing and can act as US.
1 identify a United States US Agent for that establishmentfacility. US Agent acts acts as medium of communication between FDA and foreign company. US importers must list their registered foreign manufacturers.
Food and Drug Administration FDA Center for Devices and Radiological Health CDRH received Registration and Listing information. Any foreign establishment engaged in the manufacture preparation propagation compounding or processing of a device imported into the United States must identify a United States agent US. Click the front start button for medical devices establishment registration and device listing.
FDA Registration and FDA US Agent Requirement Foreign food facilities that manufacture process pack or hold food for human or animal consumption in the US. In addition US FDA charges all medical companies an annual US FDA User Fee. The United States Food and Drug Administration FDA requires all medical device and IVD companies without a presence in the United States to appoint a registered US FDA Agent.
Foreign and domestic establishments such as a Manufactures b Initial Exporters c Initial Importers of Medical Devices intended to be sold in the USA must register and list with the FDA. FDA Agent to Non-US companies that are already in the market or intending to enter the US. The exporterimporter is not registered with FDA.
Agent Notification Receipt March 2017 The US. The appointment of a US Agent is a mandatory requirement for foreign companies who wish to sell drugs or pharmaceuticals medical devices food and dietary supplements in the United States. The US Agent is responsible for FDA questions about imported devices and helps with FDA inspection scheduling as well as communication related to the inspection.
FDA USA Agent is one of the best site that is providing Medicals Consulting Service for food registration medical device registration and others.
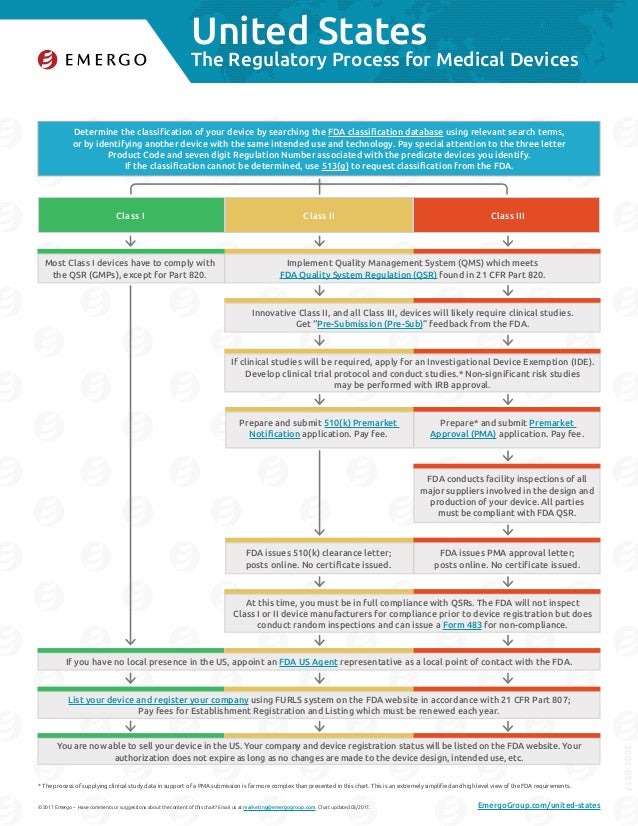
Us Fda Medical Device Approval Chart Emergo
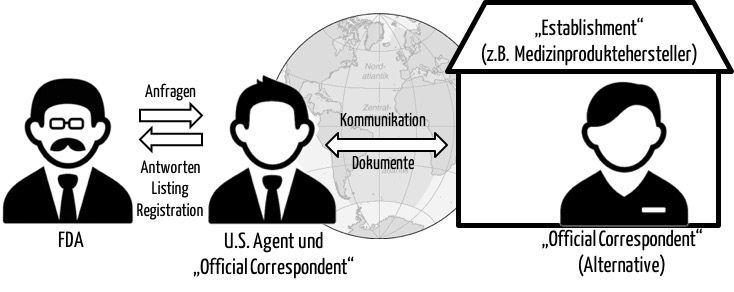
Fda U S Agent Official Correspondent Aufgaben Und Auswahlkriterien
U S Fda Medical Devices Registration And Fda Device Listing Fda Registration Assistance
Usa Regulatory Process For Medical Devices Mdrc