Types Of Medical Devices Fda
Each generic device is then assigned to one of the Classes ie. Decorative contact lenses are considered medical devices.

Fda Regulation Of Neurological And Physical Medicine Devices Access To Safe And Effective Neurotechnologies For All Americans Neuron
Premarket Notification 510 k 21 CFR Part 807 Subpart E.

Types of medical devices fda. This final rule does not change the classification of the device types for which FDA is amending the title andor identification statements. The FDA oversees their safety and effectiveness just like contact lenses that correct your vision. Specifically the FDA is amending the following classification regulations.
Use of these devices may cause serious injuries or death. Device Vibration Threshold Measurement. Speculum Vaginal Nonmetal.
All the medical device manufacturers and. The FDA has pre-determined approximately 1700 different generic types of medical devices. Device Fertility Diagnostic Proceptive.
Each of these device types is allocated to 16 different medical panels. Medical Device Classes Class I General Controls Most exempt from premarket submission Class II Special Controls Premarket Notification 510k. The Food and Drug Administration FDA is announcing the issuance of Emergency Use Authorizations EUAs the Authorizations for certain medical devices related to the Coronavirus Disease 2019 COVID-19 public health emergency.
Purifier Water Ultraviolet Medical. The FDA has identified this as a Class I recall the most serious type of recall. Calculatordata processing module for clinical use.
Cordis Corporation is recalling their Precise PRO Rx US. A medical device is used to diagnose prevent or treat a medical disease or condition without having any chemical action on any part of the body. There are 3 classes of medical devices.
Chaired by the FDA the Software as a Medical Device WG agreed upon the key definitions for Software as a Medical Device framework for risk categorization for Software as a Medical Device the. Classify Your Medical Device The Food and Drug Administration FDA has established classifications for approximately 1700 different generic types of devices and grouped them into 16 medical. Class I Class II Class III based on.
Examples include bandages handheld surgical instruments and nonelectric wheelchairs. New medical devices approved by the FDA in 2020 include incontinence treatments diagnostic testing kits and ultrasound systems used to ablate bone tumours Alongside numerous EUAs for Covid-19-related technologies a total of 48 medical device approvals have been made by the FDA in 2020 Credit. The need for this FDA ruling extends back to the 2016 passage of the 21 st Century Cures Act which introduced wide-ranging efforts to streamline market access for manufacturers of pharmaceutical products and medical devices at times by curtailing the agencys authority.
FDA has issued the Authorizations listed in this document under the Federal Food Drug and Cosmetic Act FDC Act. Here most of Class I devices are exempted from 510 k. Types of FDA Regulations for Medical Devices 1.
One of the laws provisions was to identify types of medical software that posed minimal risk to patients and absent a. Learn more about devices such as diagnostic tests ventilators and personal protective equipment PPEincluding surgical masks face shields respirators gowns and gloves. The FDA categorizes medical devices into one of three classes Class I II or III based on their risks and the regulatory controls necessary to provide a reasonable assurance of safety and.
List of Medical Devices by Product Code that FDA classifies as Implantable Life-Saving and Life-Sustaining Devices for purposes of Section 614 of FDASIA. Class I devices are low-risk devices. Establishment Registration Medical Device Listing 21 CFR Part 807.
The guidance outlines software functions that are no longer considered medical devices under the FDAs updated definitions. Plate Cranioplasty Preformed Alterable.
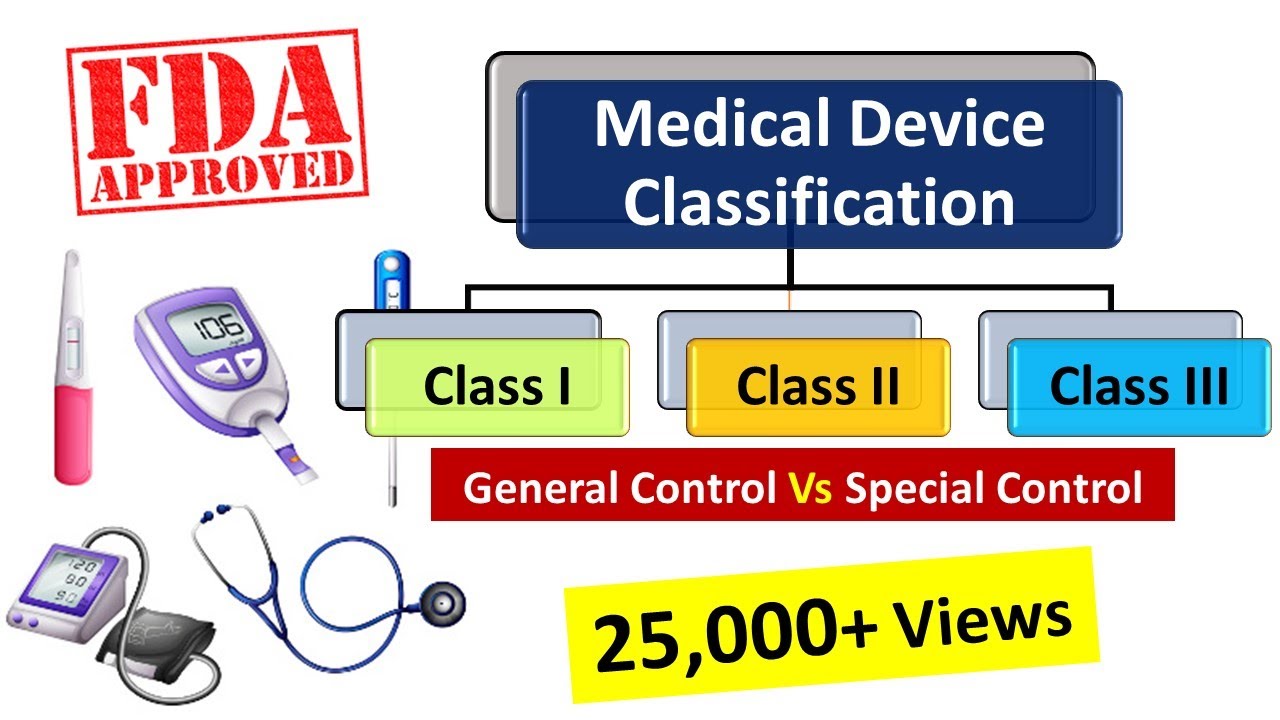
Medical Devices Classification As Per Fda Medical Device Regulations Medicaldevices Fda Youtube

Market Access For Medical Software In The United States Vde Medical Devices And Software
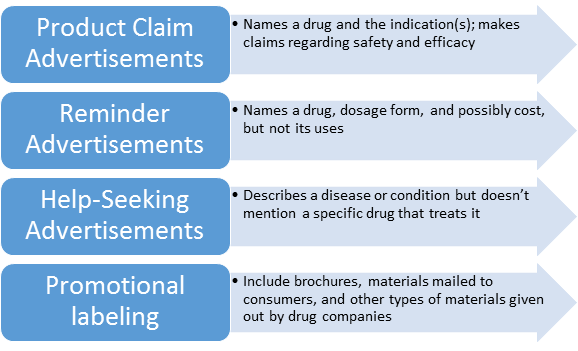
Understanding The Fda Regulations Governing Advertising And Promotion Of Drugs And Medical Devices

Fda Medical Device Classification Medical Device Validationpresentationeze