Legal Manufacturer Definition Medical Devices Fda
Lets look at the definition of a medical device. Definition of Legal Manufacturer manufacturer means the natural or legal person with responsibility for the design manufacture packaging and labelling of a device before it is placed on the market under his own name regardless of whether these operations are carried out by that person.
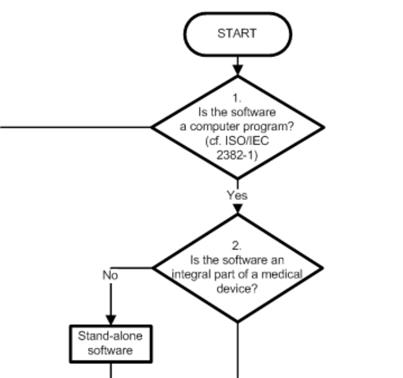
Software As Medical Device Samd Classification And Definitions
L Manufacturer means any person who manufactures prepares propagates compounds assembles or processes a device by chemical physical biological or other procedure.
Legal manufacturer definition medical devices fda. It can be found in Section 201 h of the Federal Food Drug and Cosmetic Act. B The requirement for declaration of the name of the. CFR - Code of Federal Regulations Title 21.
An instrument apparatus implement machine contrivance implant in vitro reagent or other similar or related. The FDA defines a medical device as. O Manufacturer means any person who designs manufactures fabricates assembles or processes a finished device.
Its a very broad definition. D Manufacture preparation propagation compounding assembly or processing of a device means the making by chemical physical biological or other procedures of any article that meets the definition of device in section 201h of the act. Intended for use in the diagnosis of disease or other conditions or in the cure mitigation treatment or prevention.
Legal Manufacturer means the manufacturer of a medical device in the meaning of the German Medical Devices Act Hersteller Directive 9879EC and other Applicable Laws ie. Manufacturer - Makes by chemical physical biological or other procedures any article that meets the definition of device in Section 201 h of the Federal Food Drug and Cosmetic FDC Act. FDA Updates Definition of Custom Medical Devices 30 October 2016 admin Earlier this month the US FDA updated its definition of a custom medical to include new statutory requirements for custom devices under the Federal Food Drug and Cosmetic Act the FDC Act as amended by the Food and Drug Administration Safety and Innovation Act FDASIA.
The intended performance of a device refers to the intended use for which the device is labeled or marketed as defined in 8014 of this chapter. Intended to affect the. 52 Zeilen PRE-INSPECTIONAL ACTIVITY.
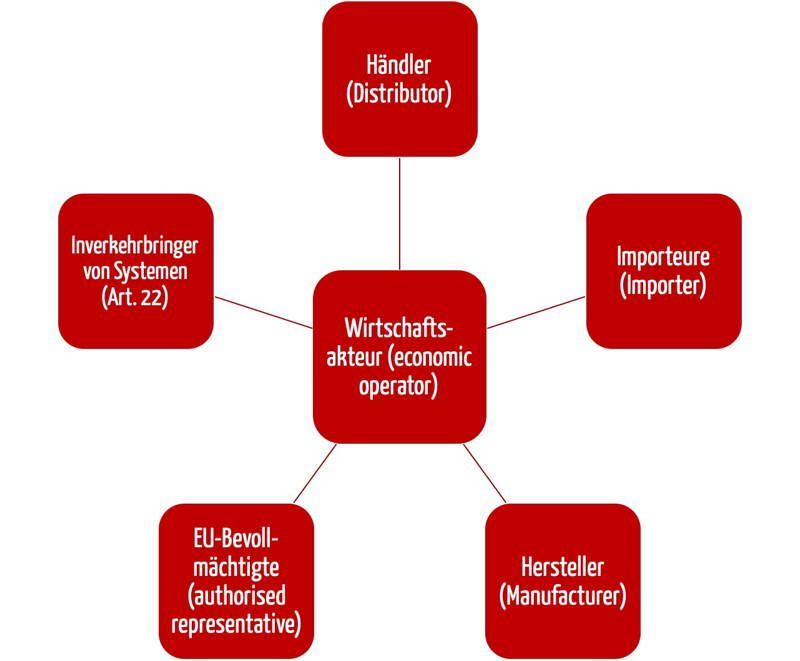
Manufacturer also includes any legal person or entity who is an. According to our consultant the legal manufacturer is always the company that produces the device and since we have contracted out the design development manufacturing to another company. T Manufacturer means any legal person or entity engaged in the manufacture of a product subject to license under the act.
Manufacturer includes but is not limited to those who perform the functions of contract sterilization installation relabeling remanufacturing repacking or specification development and initial distributors of foreign entities performing these functions. It basically says a medical device is. At our company we have a discussion about the definition of legal manufacturer for the US.
The natural or legal person who is responsible for the design manufacture packaging and labeling of a device before it is placed on the market under his own name regardless of whether these operations are carried out by that person himself or on his behalf by a Third Party. These terms include the following activities. Name and place of business of manufacturer packer or distributor.
1 medical device means any instrument apparatus appliance software implant reagent material or other article intended by the manufacturer to be used alone or in combination for human beings for one or more of the following specific medical purposes. A The label of a device in package form shall specify conspicuously the name and place of business of the manufacturer packer or distributor.

Medical Device Consultant Only A German Concept

Market Access For Medical Software In The United States Vde Medical Devices And Software

Software As Medical Device Samd Classification And Definitions