Fda Iso Standards Medical Devices
Assuring customer satisfaction. The ISO 9001 standard was entitled Quality Systems Model for Quality Assurance in Design Development Production Installation and Servicing.

Medical Device Software Iec 62304
The new lists of references of harmonised standards for medical devices have been published OJ L 0901 of 25 May 2020.

Fda iso standards medical devices. The ISO 13485 standard was entitled Quality Systems Medical Devices. New 2020 lists of harmonised standards for medical devices are now available. This goes beyond the safety and.
ISO 62304 applies to medical device software. They can be found below. Medical device companies seeking compliance with the ISO medical device standards must establish a quality management system that conforms to ISO 134852016.
Numerous regulatory agencies and standards organizations collaborate to establish the accepted standards for medical. The ISO 13485 quality standard forms the basis for quality management system requirements in the European Union Japan Canada and other medical device markets. At the same time the FDA sought to harmonize the CGMP regulations with applicable international standards.
Publications in the Federal Register to the lists of recognized consensus standards can be accessed at httpswwwfdagovmedical-devicesstandards-and-conformity-assessment. Regulatory bodies around the world including the FDA use this standard to ensure the safe design development production and maintenance of software used in medical devices. While ISO 14155 is not law in the United States it plays a role similar to ICH Good Clinical Practices Guidelines.
The ISO 14155 standards were created to clarify the design conduct recording and reporting of clinical investigations carried out in human subjects to assess the safety or performance of medical devices for regulatory purposes. The development and use of standards is vital to ensuring the safety and efficacy of medical devices. Medical Devices Medical devices are subject to strict general controls and procedural regulations.
FDA plans to issue a notice of proposed rulemaking in October 2020 establishing US quality system requirements based more closely on ISO 134852016 according to the agencys Rule List. The primary standards included International Organization for Standards ISO 90011994 and 134851996. 10079-3 Third Edition 2014-05-01.
After FDA has decided to recognize a standard we will update our online database to reflect the decision even before formal recognition of the standard occurs by publication in the Federal Register. FDA and medical device regulators from other countries see problems with enforcing some ISO 90012000 requirements relating to. ISO 14155 is in its second edition.
Greenlight Gurus eQMS software provides out-of-the-box compliance with the most current medical device quality standards including ISO 13485 and the FDA QSR. Medical suction equipment - Part 3.
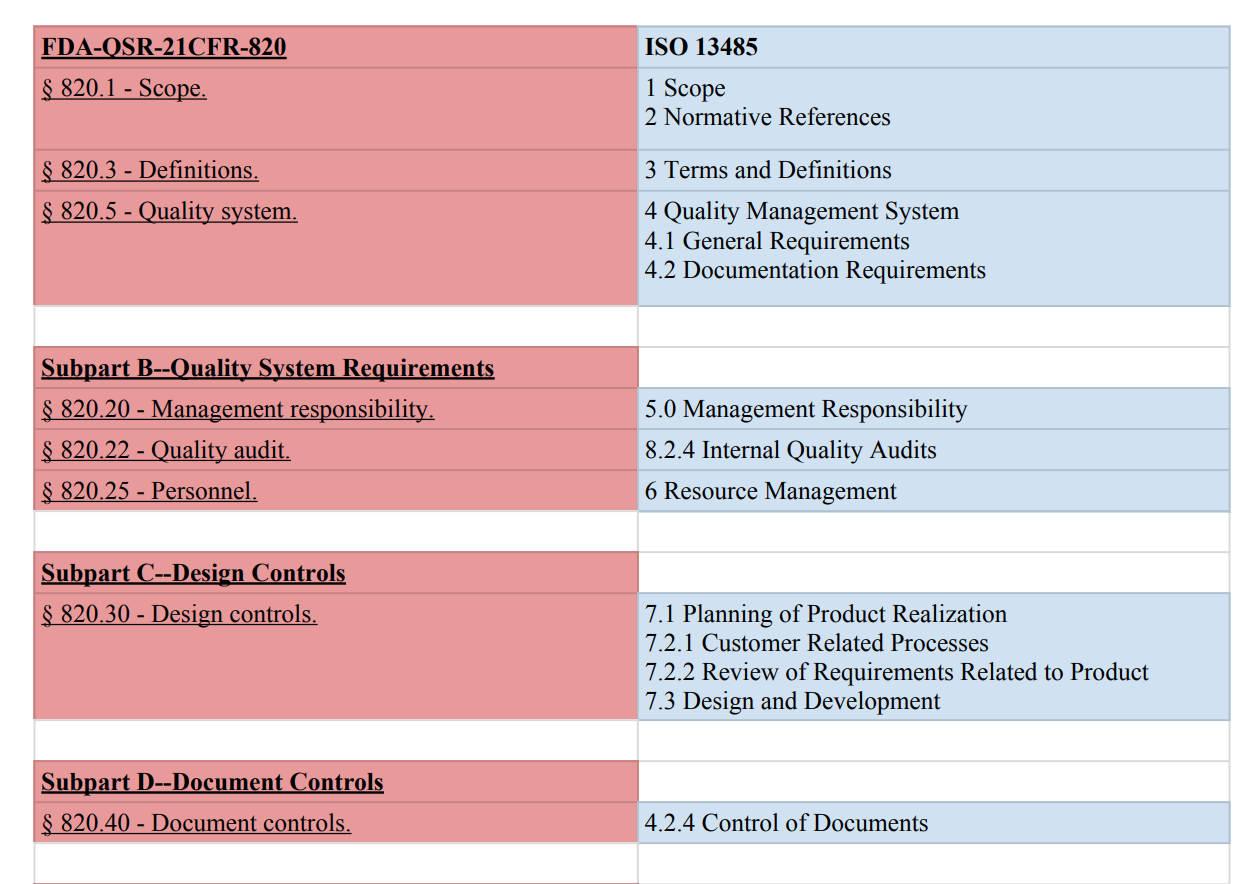
Compare Iso 13485 And Fda Qsr 21 Cfr 820 To Learn How To Transition

Medical Device Calibration A Step By Step Guide To Meeting Fda And Iso Standards Fdanews

Flow Chart From Fda Cdrh Use Of International Standard Iso 10993 Download Scientific Diagram

Alm For Medical Device Development Sdc Systems Limited