Medical Device Manufacturer Definition Fda
A medical device OEM manufacturer designs engineers and manufactures complete products and systems. Medical device contract manufacturing is the outsourced fabrication of a medical device for the medical marketplace.
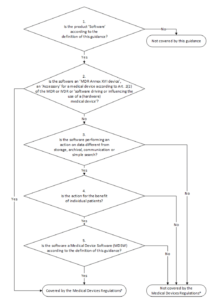
Software As Medical Device Samd Classification And Definitions
Manufacturer includes but is not limited to those who perform the functions of.

Medical device manufacturer definition fda. FDA Updates Definition of Custom Medical Devices 30 October 2016 admin Earlier this month the US FDA updated its definition of a custom medical to include new statutory requirements for custom devices under the Federal Food Drug and Cosmetic Act the FDC Act as amended by the Food and Drug Administration Safety and Innovation Act FDASIA. Preannouncements 483 Annotations Post Inspectional Correspondence. Can someone help me locate the requirements for contract manufacturing of a Medical Device.
Outside of the US. The referee test provides the Food and Drug Administration with the means of examining a medical device for performance and does not inhibit the manufacturer from using equal or superior test. Also known as a Premarket Notification a 510k notification allows the FDA to determine whether the device is equivalent to a device already placed into one of the three classification categories.
And does not achieve its primary intended action. FDC Act defines a device as. Manufacturer - Makes by chemical physical biological or other procedures any article that meets the definition of device in Section 201 h of the Federal Food Drug and Cosmetic FDC Act.
O Manufacturer means any person who designs manufactures fabricates assembles or processes a finished device. An instrument apparatus implement machine contrivance implant in vitro. Other manufacturers purchase these and assemble them into their products.
510k - One of the first steps for medical device companies who manufacture Class 2 medical devices and a small number of Class 1 and 3 devices is to file a510k with the Food and Drug Administration FDA. Decorative contact lenses are considered medical devices. For purposes of this part and in order to clarify the responsibilities of the entities engaged in different operations the term manufacturer is defined and used separately from the terms.
Our company is a 90012015 134852016 registered engineering and build to print firm. We only carry the certificate to help understand the needs of capital equipment customers who use the automation cells we build to produce medical. We have taken an exclusion to 13485 - 73 as we do not design or build medical devices.
The FDA oversees their safety and effectiveness just like contact lenses that correct your vision. Definition of a Medical Device. Some OEMs also manufacture sub-assemblies or component parts.
A set of very similar regulations nearly exactly. Section 201h of the Food Drug Cosmetic Act. Design controls for medical devices are regulated by the FDA under 21 CFR 82030.
L Manufacturer means any person who. The intended performance of a device refers to the intended use for which the device is labeled or marketed as defined in 8014 of this chapter. We specialize in capital equipment but have also done end product in low volumes.
They must be implemented by manufacturers of class II or III medical devices and some class I devices. 52 Zeilen PRE-INSPECTIONAL ACTIVITY. A medical device is formally defined by the World Health Organization as any instrument apparatus implement machine appliance implant reagent for in vitro use software material or other similar or related article intended by the manufacturer to be used alone or in combination for human beings for one or more specific medical purpose.
Medical devices are defined by the US Food and Drug Administration FDA as any object or component used in diagnosis treatment prevention or cure of medical conditions or diseases or affects body structure or function through means other than chemical or metabolic reaction in.

What Is A Medical Device Official Definition For Eu Usa China Brazil

Device Regulations The New Medical Device Regulation The Applicability Of Article 117 To Medicinal Products

What Is A Medical Device Official Definition For Eu Usa China Brazil

Software As Medical Device Samd Classification And Definitions