Legal Manufacturer Medical Device Definition
1 medical device means any instrument apparatus appliance software implant reagent material or other article intended by the manufacturer to be used alone or in combination for human beings for one or more of the following specific medical purposes. Of the conformity assessment.
If you make a medical claim for your product chances are good that it is a medical device and taking a very close look at the legally binding definition is indispensable.

Legal manufacturer medical device definition. Legal Manufacturer means the manufacturer of a medical device in the meaning of the German Medical Devices Act Hersteller Directive 9879EC and other Applicable Laws ie. An Own Brand Labeller OBL purchases a finished or component parts of a medical device from the Original Equipment Manufacturer OEM which he then places on the market under his own name or trade mark brand label. The natural or legal person who is responsible for the design manufacture packaging and labeling of a device before it is placed on the market under his own name regardless of whether these operations are carried out by that person himself or on his behalf by a Third Party.
Medical device software is software that is intended to be used alone or in combination for a purpose as specified in the definition of a medical device in the MDR or IVDR regardless of whether the software is independent or driving or influencing the use of a device. Identifie d as legal manufacturer on t he label the fulfilment. A natural or legal person who manufactures or fully refurbishes a device or has a device designed manufactured or fully refurbished and markets that.
Who is the manufacturer. For manufacturers of medical devices ie. Manufacturer also includes any legal person or entity who is an.
For the purposes of this Regulation the following definitions apply. This Own Brand Labeller may not be the person who actually designs manufactures packages or labels the device. Existing medical devices that will not be upgraded to compliance with the EU MDR by virtue of the transitional provisions are known as legacy devices.
In accordance with the Medical Devices Regulation EU 2017745 a manufacturer is defined as. The manufacturer of a medical device is the person who is responsible for the design production packaging and labelling of the device before it is supplied under the persons name whether or not it is the person or another person acting on the persons behalf who carries out those operations. Manufacturer - Makes by chemical physical biological or other procedures any article that meets the definition of device in Section 201h of the Federal Food Drug and Cosmetic FDC Act.
Means the natural or legal person with responsibility for the design manufacture packaging and labelling of a medical device before it is placed on the market under his own name regardless of whether these operations are carried out by that person himself or on his behalf by a third party. One of the conditions for benefiting from the transitional provisions is that the legacy device does not undergo significant changes in design or. T Manufacturer means any legal person or entity engaged in the manufacture of a product subject to license under the act.
Manufacturer means the natural or legal person with responsibility for the design manufacture packaging and labelling of a device before it is placed on the market under his own name regardless of whether these operations are carried out by that person himself or on his behalf by a third parry. Medical device means any instrument apparatus implement machine appliance implant reagent for in vitro use software material or other similar or related article intended by the manufacturer to be used alone or in combination for human beings for one or more of the specific medical purpose s of. The legal frameworks MDD as well as MDR provide definitions of medical devices which in theory should make it possible to qualify products as medical devices.
Define Manufacturer of a medical device. Procedure chosen by the manufacturer is also taken into account. Manufacturer means a natural or legal person who manufactures or fully refurbishes a device or has a device designed manufactured or fully refurbished and markets that.
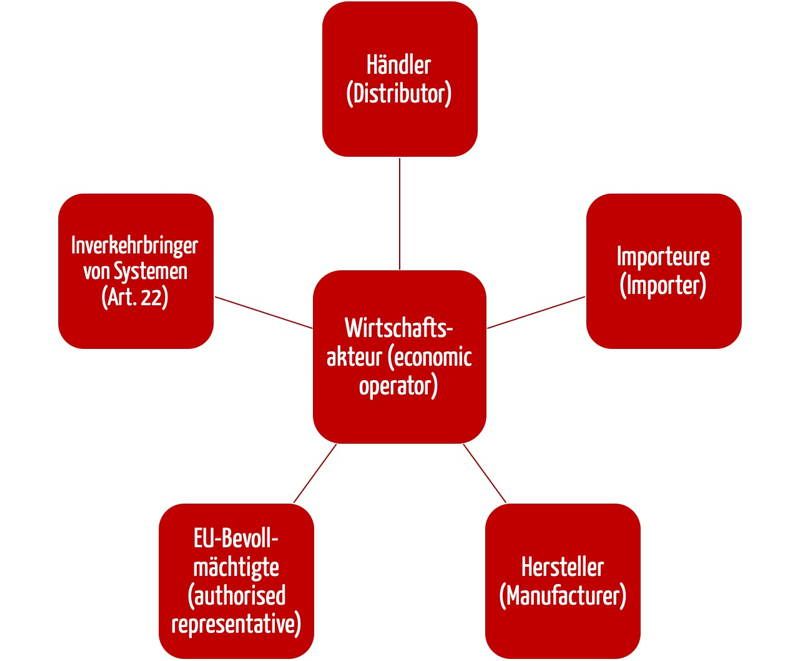
Anforderungen An Handler Die Auch Die Hersteller Betreffen
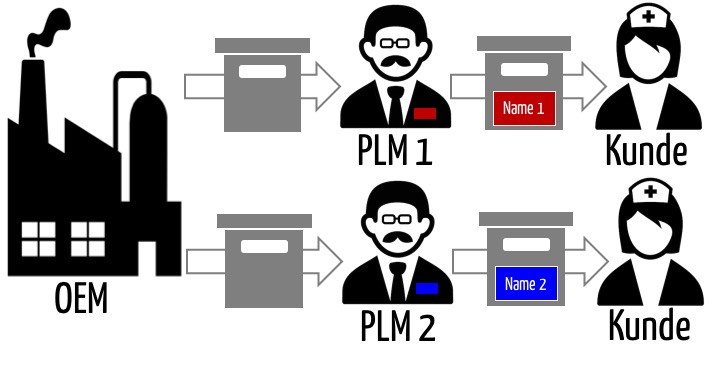
Oem Original Equipment Manufacturer Von Medizinprodukten
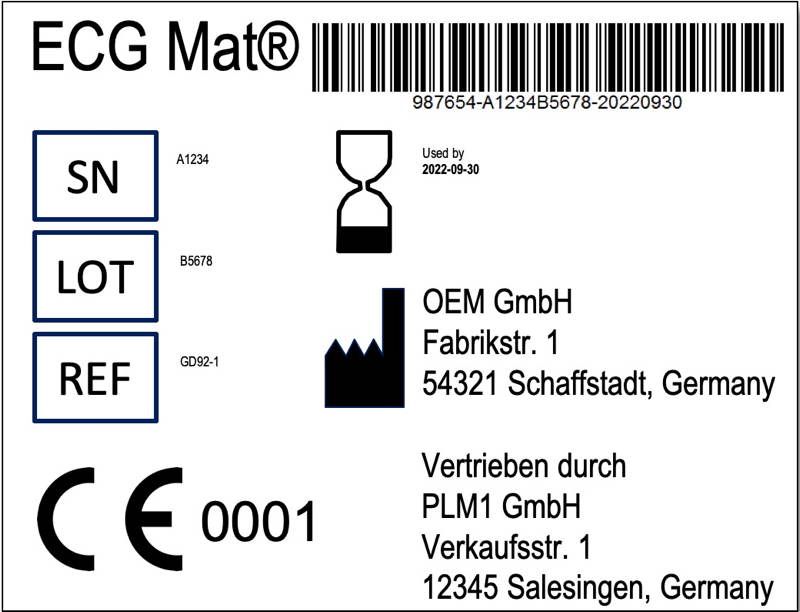
Anforderungen An Handler Die Auch Die Hersteller Betreffen

Taxonomy Of Big Data Technology Google Search In 2021 Big Data Technologies Taxonomy Technology
