Fda Pest Control Medical Devices
FDA authority applies to the finished device manufacturer Outsourcing of critical components and manufacturing of medical devices FDA does not perform routine. We prepare a comprehensive pest management plan specifically for your facility that utilizes the latest industry tools and environmentally responsible techniques.
For the most up-to-date version of CFR Title 21 go to the Electronic Code of Federal Regulations eCFR.

Fda pest control medical devices. In a single direction in a robust and uniform. Design Controls are a systematic framework for capturing key aspects of medical device product development to prove your product meets user needs and is safe and effective. CHAPTER I--FOOD AND DRUG.
21CFR111 TITLE 21--FOOD AND DRUGS CHAPTER I. Special care and consideration. Most medical devices interact with the human body during the surgical process.
Draft for Comments of Guidelines for the Use of the Food and Drug Administration FDA eServices Portal System for License to Operate LTO Application of Traders and Distributors including Wholesalers Importers and Exporters of Medical Devices Equipment or Devices Used for Treating Sharps Pathological and Infectious Waste and Water Treatment DevicesSystems. For the most up-to-date version of CFR Title 21 go to the Electronic Code of Federal Regulations eCFR. Manner and at sufficient speed to.
Help More About 21CFR. They are also charged with ensuring biological devices and medical devices intended for. Program emphasizes prevention as the primary pest management method for your hospital or clinic.
Minimum sample size - Guidance and statistical rationale. Help More About 21CFR Code of Federal Regulations Title 21 Volume 2 Revised as of April 1 2020 CITE. Pest Control for Medical Facilities A Diagnostic Approach to Controlling Pests.
Risk Management There are references to risk management in FDA 82030 and ISO 13485. 101-629 enacted on November 28 1990 amended section 520f of the act providing FDA with the authority to add preproduction design. Databases - The information on this page is current as of April 1 2020.
AAMI TIR45 guidance wanted. E Each freezer and cold storage compartment used to store and hold food capable of supporting growth of microorganisms shall be fitted with an indicating thermometer temperature-measuring device or temperature-recording device so installed as to show the temperature accurately within the compartment and should be fitted with an automatic control for regulating temperature or with an automatic alarm. The Food and Drug Administration FDA is responsible for protecting public health by assuring foods cosmetics dietary supplements and drugs are safe wholesome sanitary and properly labeled.
Databases - The information on this page is current as of April 1 2020. Medical Device and FDA Regulations and Standards News. For the most up-to-date version of CFR Title 21 go to the Electronic Code of Federal Regulations eCFR.
Reproducibly sweep particles away from the. Help More About 21CFR Code of Federal Regulations Title 21 Volume 8 Revised as of April 1 2020 CITE. TITLE 21--FOOD AND DRUGS CHAPTER I--FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES SUBCHAPTER H - MEDICAL DEVICES.
The Safe Medical Devices Act of 1990 the SMDA Pub. Contact FDA Follow FDA on Facebook Follow FDA on Twitter View FDA videos on YouTube Subscribe to FDA RSS feeds FDA Homepage Contact Number 1-888-INFO-FDA 1-888-463-6332. Then all of those medical devices would transfer physical debris weighing in micrograms into the exposed human body during the surgical process or transfer biological agents into the body ultimately causing various serious.
21CFR82070 TITLE 21--FOOD AND DRUGS. Just imagine if we didnt have a cleaning process or sterilization process in place. FDA guidance on non-sterile Medical Device Packaging.
The Food and Drug Administration FDA is responsible under the Federal Food Drug and Cosmetic Act for enforcing tolerances established by the Environmental Protection Agency EPA. FDA Guidance definition An airflow moving. Databases - The information on this page is current as of April 1 2020.
Medical Device and FDA Regulations and Standards News. Here is an overview of the FDA regulations concerning medical devices. Inspection Prints Drawings Testing Sampling and Related Topics.

Food Drug Device Fda Environment Land Use Natural Resources Advisory Cleaning Up After Covid 19 What You Need To Know Before You Manufacture Import Or Sell Sterilizers Sanitizers Purifiers And Disinfectants

Pest Control Program Gmp Sop Standard Operation Procedure
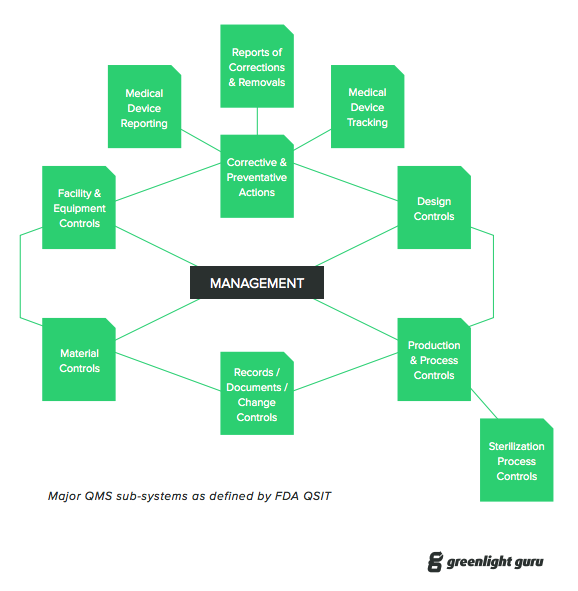
Iso 13485 And Fda Qsr A Step By Step Guide To Complying With Medical Device Qms Requirements
