Fda Medical Device Listing Database
Registration of a device establishment assignment of. Can assist you in deciding if FDAs UDI is required for your device as well as submitting your device information to FDAs Global Unique Device Identification Database GUDID.

How To Search Fda Registration Number Fdabasics
Because the listing of products can be.

Fda medical device listing database. Establishment Registration and Medical Device Listing Files for Download Releasable establishment registration and listing information under the Freedom of Information Act is available by searching. More resources on US FDA medical device. There are still ways that device makers can avoid submitting individual injuries and malfunctions to the MAUDE database.
Learn more about devices such as diagnostic tests ventilators and personal protective equipment PPEincluding surgical masks face shields respirators gowns and gloves. All Rights Reserved for Saudi Food and Drug Authority 2021 The site supports all browsers as well as all smart devices. 10903 New Hampshire Avenue Silver Spring MD 20993 Ph.
Medical Device FDA Registration Number and Device Listings. You can use AccessGUDID to. Medical device manufacturers registered with FDA and.
Select Product Codes Select a product code for the new device listing. In that case you can contact us for immediate FDA food facility registration assistance. Medical Image Storage Device LMB NFF.
The Global Unique Device Identification Database GUDID contains key device identification information submitted to the FDA about medical devices that have Unique Device Identifiers UDI. Picture Archiving and Communications System QIH OMJ NWE PGY OEB QKB PZO NFJ LLZ. 1-888-INFO-FDA 1-888-463-6332 Contact FDA.
26 Zeilen Medical Device Databases. A listing of all device product codes associated with your account will be displayed. Can assist you in deciding if FDAs UDI is required for your device as well as submitting your device information to FDAs Global Unique Device Identification Database GUDID.
US Food and Drug Administration. Drug Listing NDC Number Search. Click the start button for free initial assessment and GUDID submission and UDI assistance.
Medical Image Communications Device NFG LMD. Home Uterine Activity Monitor LQK MOH. This database contains key device identification information submitted to the FDA about medical devices that have Unique Device Identifiers UDI.
The FDA is establishing the unique device identification system to adequately identify devices sold in the US- from manufacturing through distribution to patient use. FDA has maintained the database for medical device and drug establishment registrations whereas there is no online searchable database for food facility registration. For other industries you can use the following links to access FDA registration data.
Food Facility Registration number. Knowing where devices are. To replace the ASR.
Drug Establishment FDA Registration Search. Here are the step by step instructions to search FDA Registration Number 1. A list of all medical devices with their associated classifications product codes FDA.
Export Device to the United States But Perform No Other Operation on Device. Medical Device 510k Number Search. This database includes.
FDA also requires Medical Device labelers to submit certain information about each device including UDI to FDAs Global Unique Device Identification Database GUDID. Medical devices listed with FDA. Medical Device Data System OUG.
Registration and listing provides FDA with the location of medical device establishments and the devices manufactured at those establishments. Click the start button for free initial.
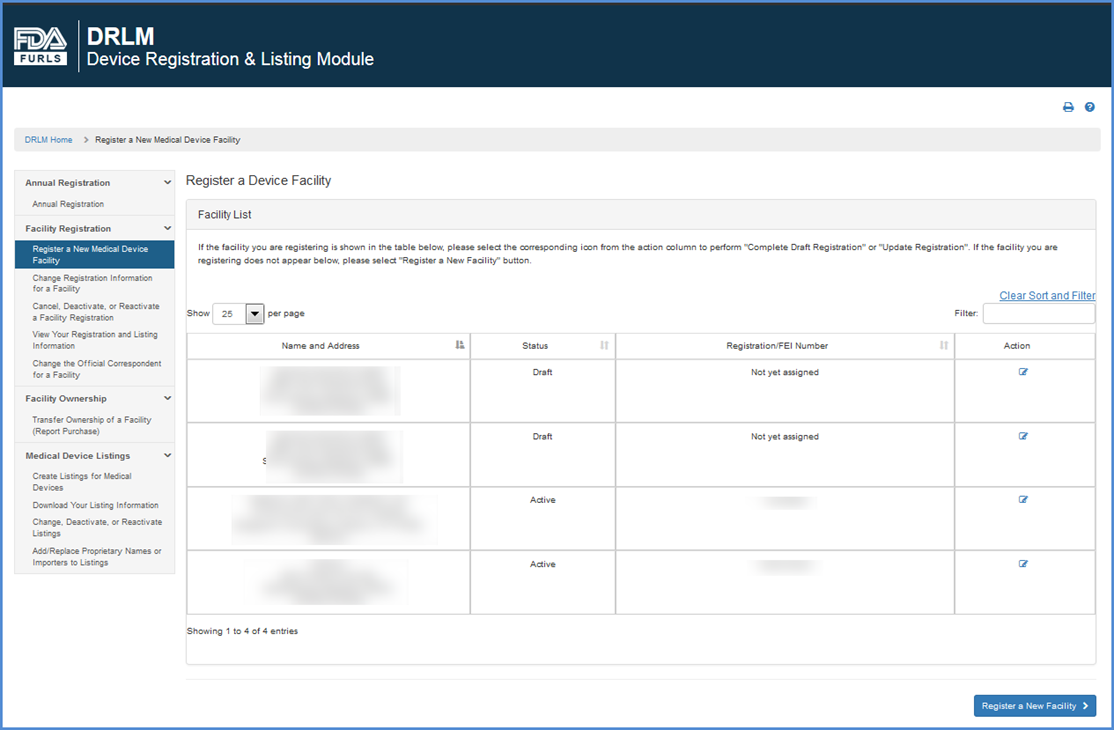
Register A New Medical Device Facility Step By Step Instructions
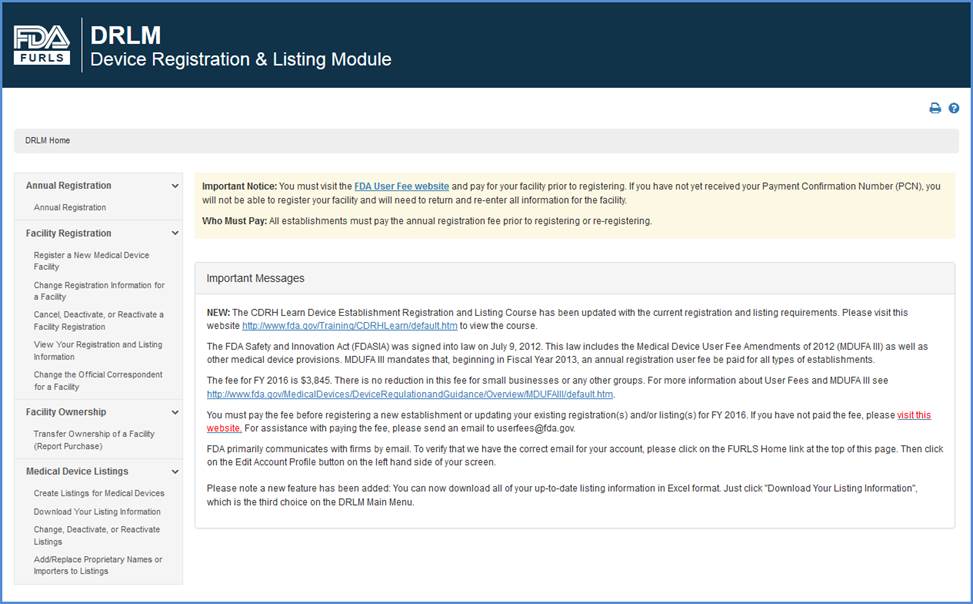
Device Registration And Listing Module Drlm Step By Step Instructions
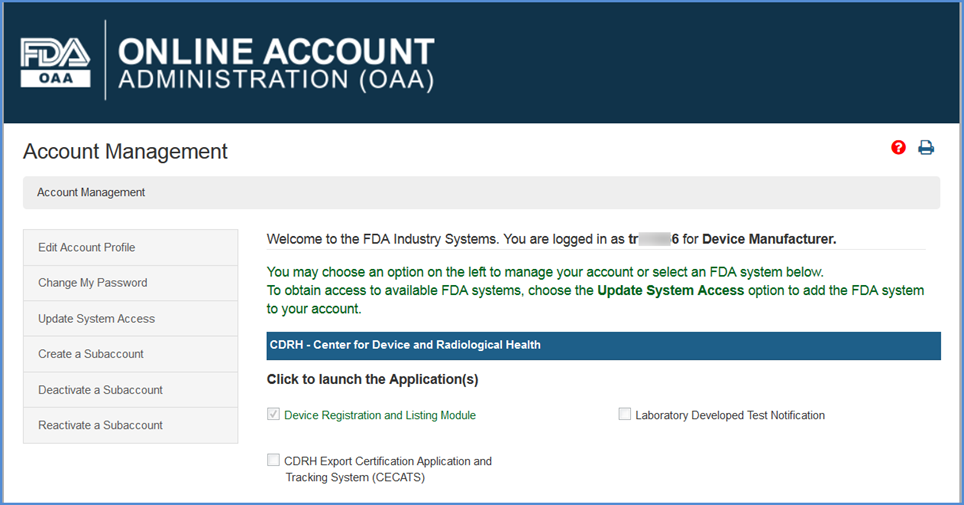
Device Registration And Listing Module Drlm Step By Step Instructions
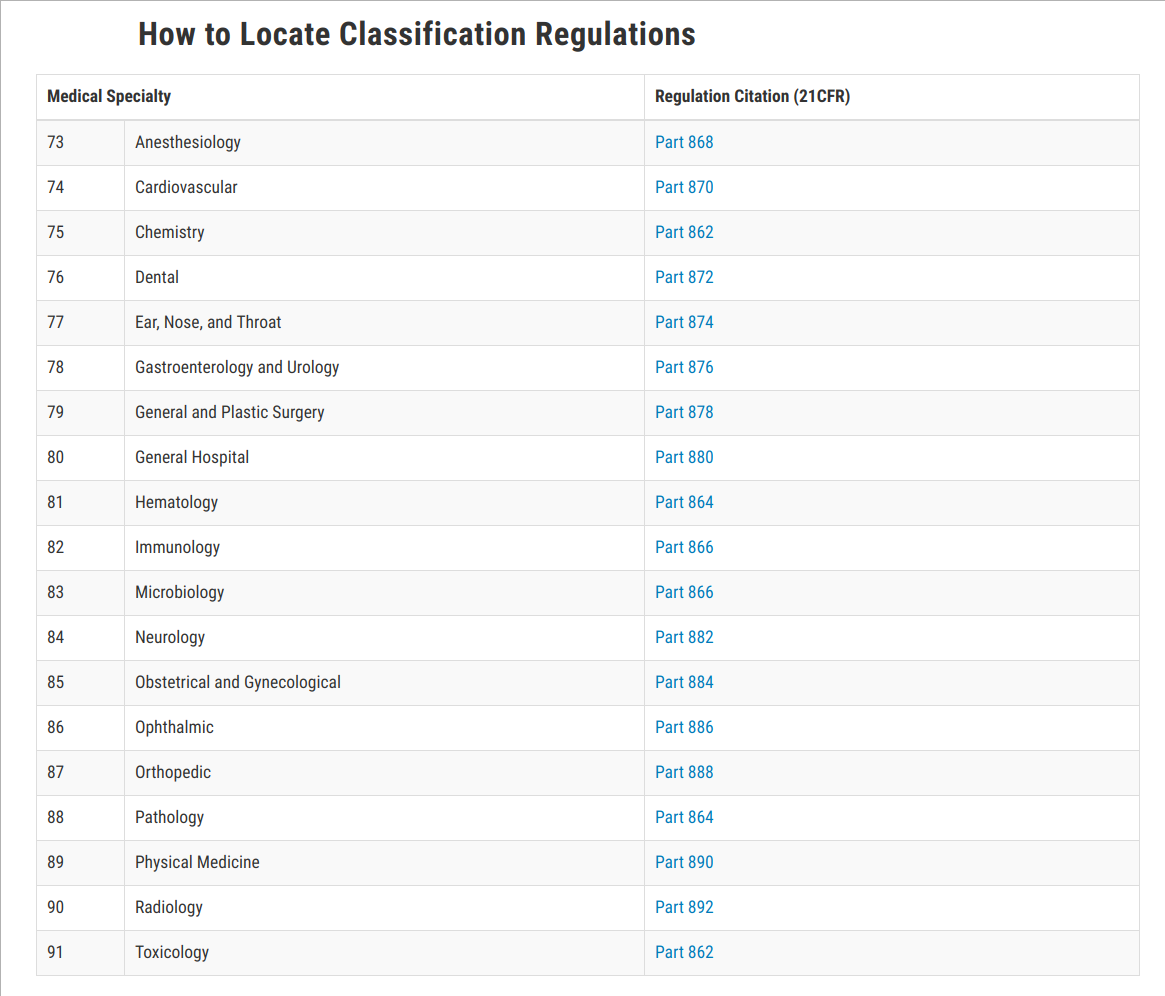
Does An Fda Class 1 Medical Device List Exist