Fda Approval Process For Medical Devices Ppt
The pathway to approval for a medical device depends on its risk classification. The FDA has 90 days to audit your 510k application after you submit it.
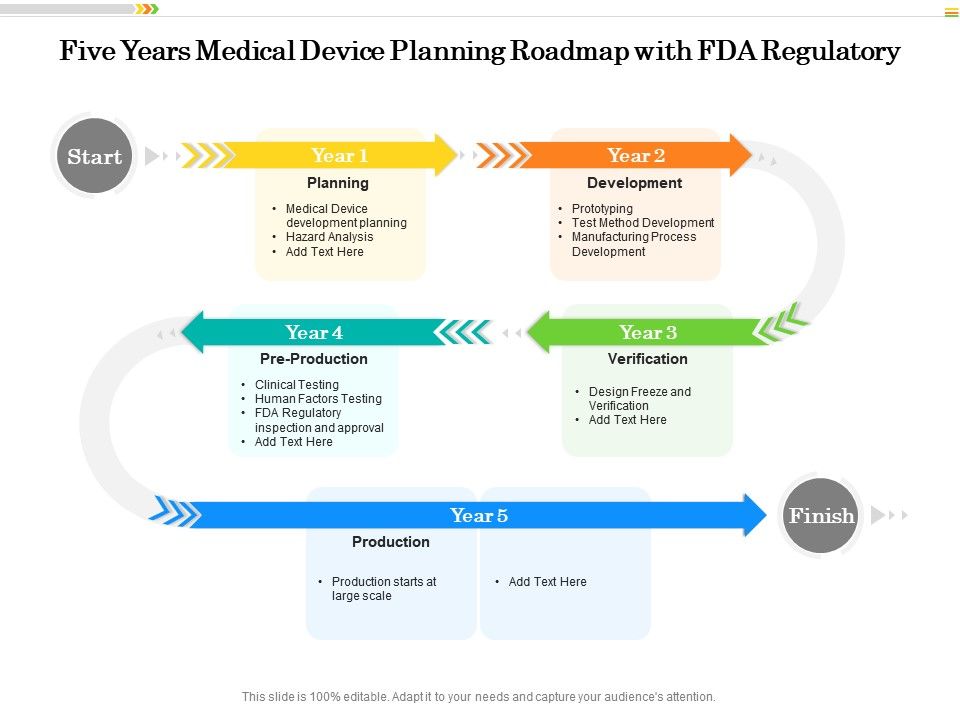
Five Years Medical Device Planning Roadmap With Fda Regulatory Presentation Graphics Presentation Powerpoint Example Slide Templates
Process 1 Decide the classification of your device by examining the FDA classification database using relevant search terms or by distinguishing another device with the equivalent planned use and innovation.

Fda approval process for medical devices ppt. The US FDA medical device IVD approval process explained. However medical devices are used in real-time where it is imperative for equipment to perform without any risk of failure or hazard. 1 the PMA process.
Welcome to FDAs information about medical device approvals. Overview of regulations for medical devices. Recently Approved Devices that include some of the newest medical technology available.
Grant special attention to the three-letter Product Code and seven-digit Regulation Number related to the predicate devices you. If your medical device is cleared. Access to Safe and Effective Neurotechnologies for All Americans.
FDA Regulation of Neurological and Physical Medicine Devices. Because there is so much variation in the classification of devices developers have a. Premarket notifications 510k establishment registration device listing quality systems labeling and reporting requirements.
Neuron Volume 92 Issue 5 943 - 948. To get FDA approval for your medical device youll need to go through the following five steps. The FDA approval process for medical devices is not as stringent as for medicines or drugs.
You should know your devices classification before the development process begins. The following information is available. Safe Medical Devices Act SMDA of 1990 FDA Modernization Act FDAMA of 1997 Medical Device User Fees Acts of 2002 2007.
It consists of the Office of the Commissioner. During the audit they will likely reach out to you for additional product information at which time the 90-day clock is stopped and restarted upon the FDAs receipt of the requested information. FDA Overview The Food and Drug Administration FDA is an agency within the US.
Introduction to US FDA Medical Device Regulatory Process. Anderson Leigh et al. 2 the PMN process.
Department of Health and Human Services. Know Your Devices Classification. There are 3 basic processes to obtain FDA marketing approval for medical devices depending on the nature of the device and the circumstances under which approval is sought.
3 Basic Pathways to Medical Device Approval. Interested in learning more about the process to. Step 1 Determine the classification of your medical device or in vitro diagnostic IVD device by searching the FDA classification database using relevant search terms or by identifying another predicate device with the same intended use and technology.
How long does it take to finalize the FDA approval process for medical devices. Approval Determining Device Classifications Investigational Device Exemption IDE for clinical studies Quality system regulation and labeling requirements Medical device and adverse event reporting 3. 3 Definition of a Device 201h an instrument apparatus implement.
New Drug Approval Process FDA CDER Center for Drug Evaluation and Research Clinical Trials 2. The United States is the number one medical device market in the world accounting for more than 40 of all healthcare spending worldwide. Pay special attention to the three-letter Product Code and seven.
And 3 the humanitarian device exemption HDE process. The US has 310 million people and the highest per-capita spending on healthcare worldwide. FDA medical device approval process step-by-step guide.
The class of device will correlate to how. Since medical devices can be basic such as the general equipment used in the hospitals clinics etc or advanced devices that are specifically used during operations. FDAs authority to regulate medical devices originates from the Medical Device Amendments to the Federal Food Drug and Cosmetic Act or FDC Act of 1976.
New drug approval process 1. The FDA is currently developing draft guidance for public comment to help industry and FDA staff understand how the 21 st Century Cures Act affects FDAs oversight of medical device software.
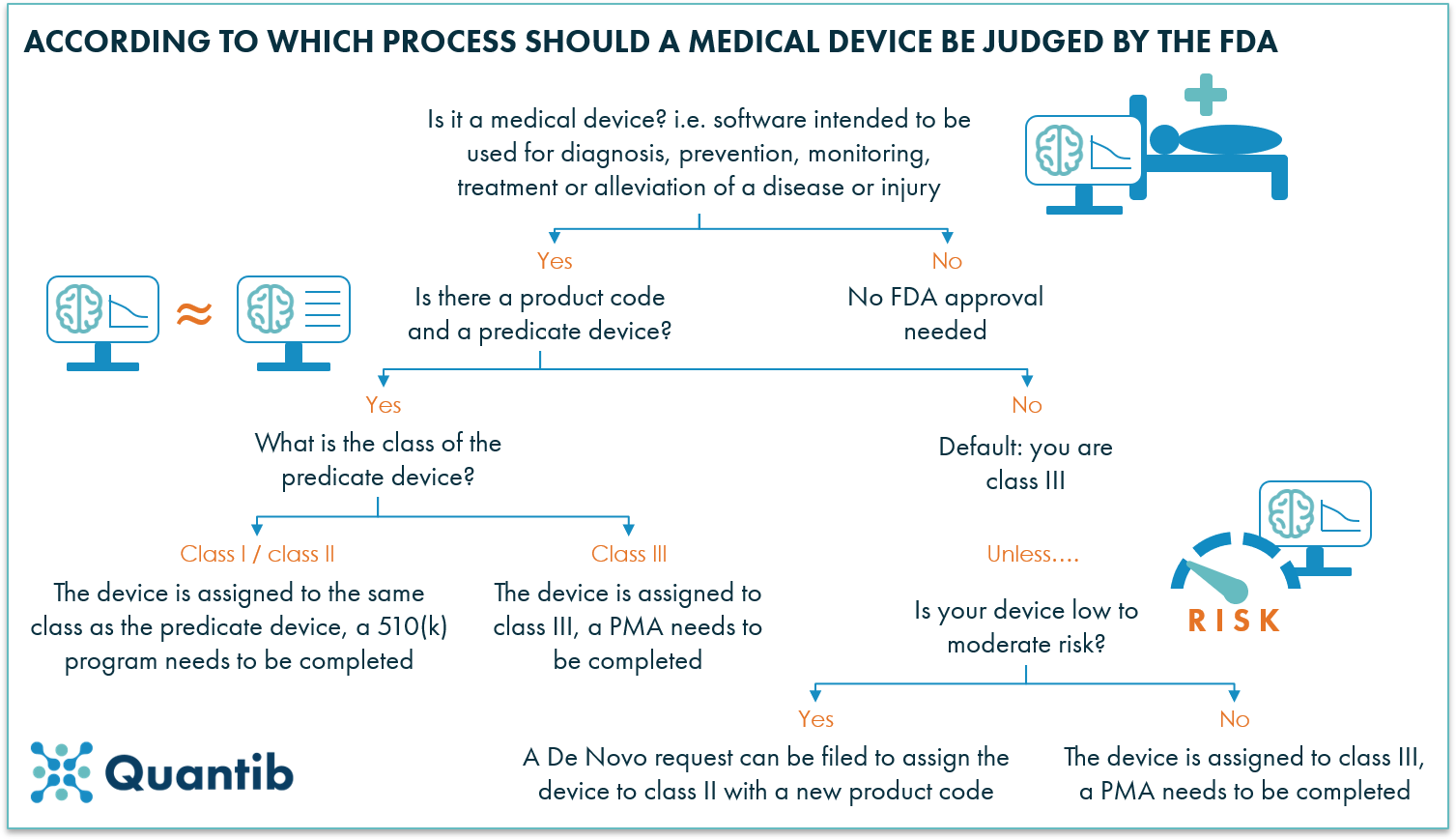
A 101 Guide To The Fda Regulatory Process For Ai Radiology Software
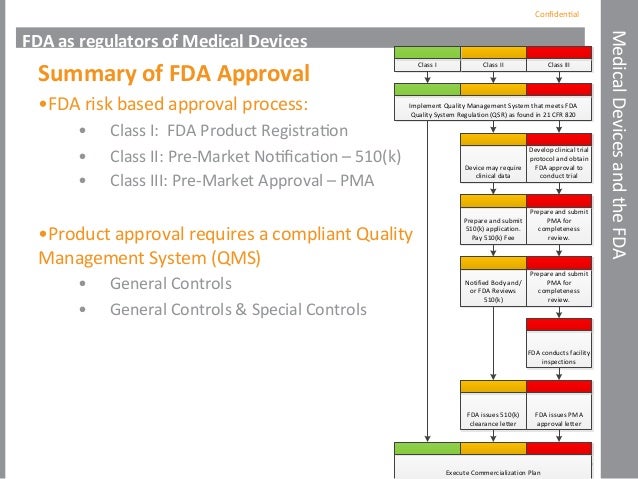
Fda Regulations And Medical Device Pathways To Market

Five Years Roadmap Of Medical Device Approval From Fda Regulatory Powerpoint Slides Diagrams Themes For Ppt Presentations Graphic Ideas
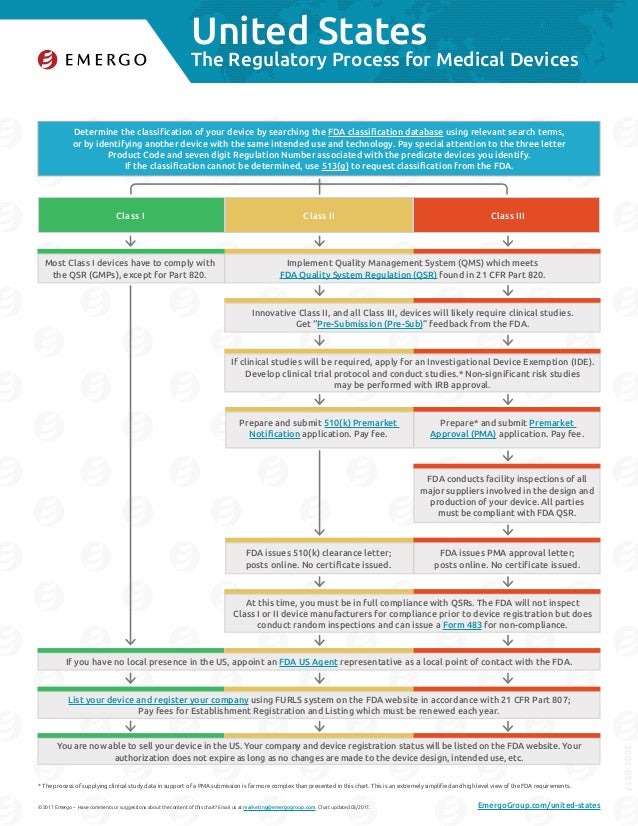
Us Fda Medical Device Approval Chart Emergo