Fda Medical Device Class 1 Definition
8805140 - Pediatric medical crib. The potential patient impact could be.
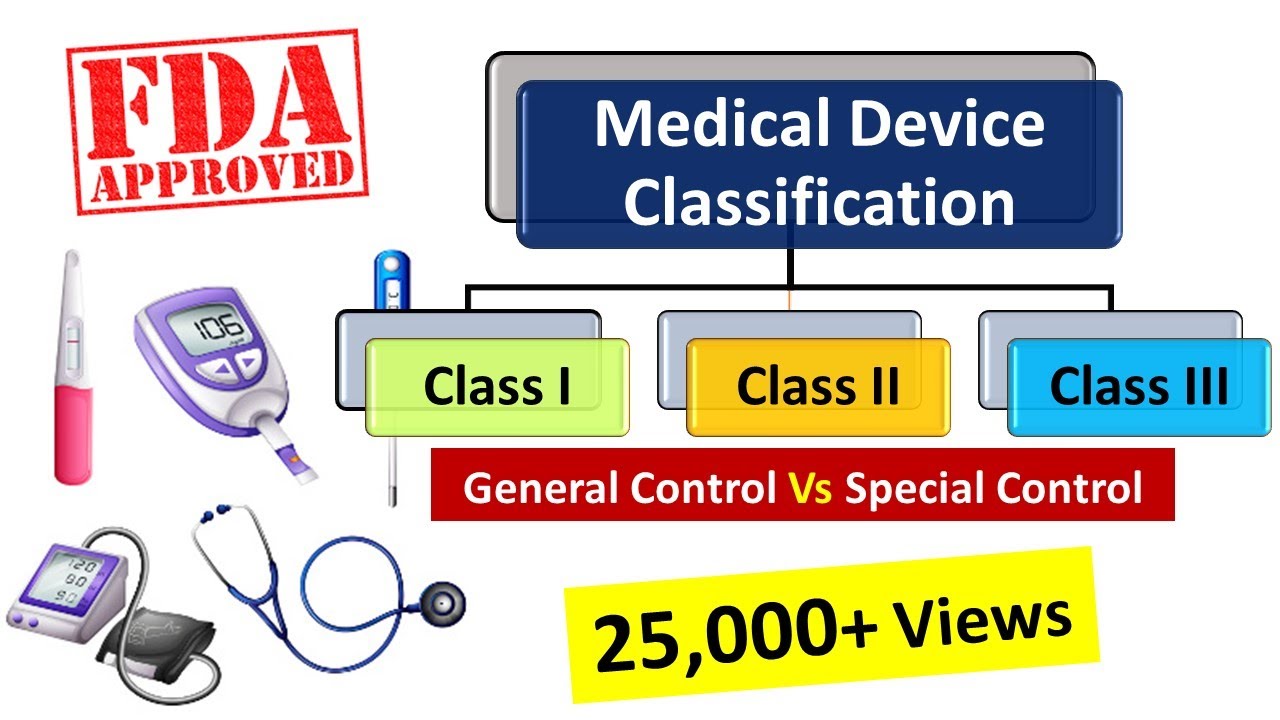
Medical Devices Classification As Per Fda Medical Device Regulations Medicaldevices Fda Youtube
8805240 - Medical adhesive tape and adhesive bandage.

Fda medical device class 1 definition. The class to which your device is assigned determines among other things the type of premarketing submissionapplication required for FDA clearance to market. This slide describes the device classification. Class 1s Medical Device Sterile Medical Devices Class 1s medical devices have lowmedium risks perceived.
Currently in the Code of Federal Regulations there are about 1700 devices. Electrical failures were identified in cardioverter defibrillators ICDs due to damaged aluminum wires. A manual toothbrush is 510 k exempt meaning it does not require premarket.
Class I These devices present minimal potential for harm to the user and are often simpler in design than Class II or Class III devices. 8805160 - Therapeutic medical binder. The device class will be displayed as 1 2 or 3.
The device classification regulation defines the regulatory requirements for a general device type. In addition for class 1 medical devices the MDR does not insist on the certification of the quality management system by a notified body. The UPC will serve as the unique device.
In this example a manual toothbrush is a class 1 medical device. A Class I or Class II device that is exempt from 510 k requirements must still comply with other requirements known as regulatory controls unless the device is explicitly exempt from those. ICDs may contain electrical wire connections which may not be completely insulated.
In this case of devices placed on the market in sterile condition the Notified Body involvement is limited to the aspects of manufacture concerned with securing and maintaining sterile condition. Regulatory control increases from Class I to Class III. Both these things save time and money.
Medical device software is software that is intended to be used alone or in combination for a purpose as specified in the definition of a medical device in the MDR or IVDR regardless of whether the software is independent or driving or influencing the use of a device. Class 1m Measuring Devices. 8805180 - Burn sheet.
Examples include enema kits and elastic bandages. Class I includes devices with the lowest risk and Class III includes those with the greatest. A medical devices class determines what controls have been put in place to manage risk and assure patients doctors and manufacturers that the device will operate safely and effectively.
8805150 - Nonpowered flotation therapy mattress. FDAs introduction to its rules for medical device regulation states. Devices that are placed on the market in sterile.
In the United States the FDA has the authority to regulate medical devices before and after they reach the marketplace. FDA has classified all medical devices into either Class I II and III. 8805200 - Intravascular catheter.
8805210 - Intravascular catheter securement device. A notified body does not have to be involved in the conformity assessment procedure for class 1 medical devices. D A class I device that bears a Universal Product Code UPC on its label and device packages is deemed to meet all requirements of subpart B of this part.
Class 1s 1r and 1m medical devices are exceptions to this rule. The FDA categorizes medical devices into one of three classes Class I II or III based on their risks and the regulatory controls necessary to provide a reasonable assurance of safety and. 8805145 - Medical bassinet.
Medical devices are classified into Class I II and III. Class 1 Device Recall Ellipse ICD. Provides definition of a medical device FDA classification panels conducted initial classification of preamendments medical devices ie Class I II III.
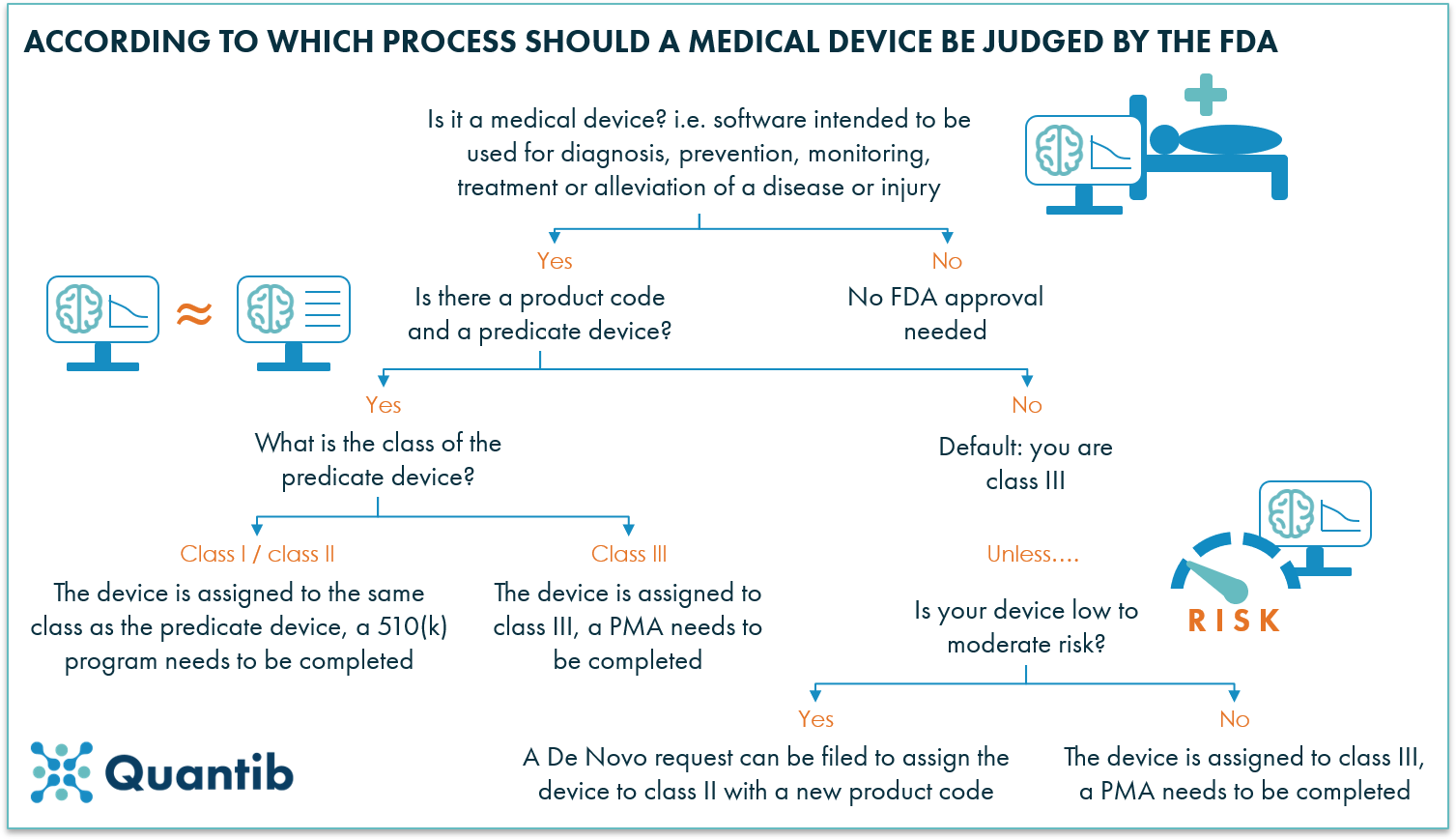
A 101 Guide To The Fda Regulatory Process For Ai Radiology Software
Fda 101 For Medical Devices Youtube

Market Access For Medical Software In The United States Vde Medical Devices And Software

Fda Medical Device Classification Medical Device Validationpresentationeze