Fda Guidance Labeling Regulatory Requirements For Medical Devices
On June 15 2016 the Food and Drug Administration FDA published a final rule 81 FR 38911 Use of Symbols in Labeling. Providing Regulatory Submissions for Medical Devices in Electronic Format Submissions Under Section 745A b of the Federal Food Drug and Cosmetic Act.

The Definitive Guide To Ifu For Medical Devices
This document will describe the requirements that must be followed for medical device manufacturing in the US.

Fda guidance labeling regulatory requirements for medical devices. This information includes the regulations governing the medical device and the pathway for. This guidance is to be used in the preparation of labelling material for non- in vitro diagnostic devices. 13 Scope and Application.
These labeling requirements rolled out to implantable life-saving and life-sustaining medical devices first and gradually expanded to the rest of the industry. Medical device reporting reporting of medical device-related adverse events 21. Labeling regulations pertaining to medical devices are found in the following Parts of Title 21 of the Code of Federal Regulations.
Must comply with are. By September 24 2020 every type of device will require UDI labeling including labels on the devices themselves for reusable items that. The final rule revises FDA labeling requirements of medical devices and certain biological products concerning the inclusion of symbols or graphical representations of information.
Medical Device Label and Labeling Introduction to Medical Device Labeling Label vs. The basic regulatory requirements that manufacturers of medical devices distributed in the US. Labeling - Regulatory Requirements for Medical Devices GPO 017-012-00327-3 275 PB 86-184348AS 1195.
This publication explains label and labeling regulations and requirements for medical devices. Medical devices offered or imported for sale or use in Canada must meet the labelling requirements listed in sections 21 - 23 of the Regulations. The FDA bases regulations for medical devices on a device classification and product code system.
HHS and FDA remind regulated entities that various requirements under the FDC Act and FDA regulations apply to the class I. 2 Every device package shall bear a UDI. HHS and FDA are concerned about the public health risks posed by the January 15 2021 Notice particularly as the Notice applies to medical gloves that could pose undue risk as described in the Gloves PHE Guidance.
This draft guidance document provides Food and Drug Administrations recommendations on testing to assess the safety and compatibility of medical devices in the Magnetic Resonance MR Environment. 80130 - General exceptions from the requirement for the label of a device. You must comply with all the FDC Acts requirements including but not limited to.
The FDA reviewed the IpsiHand System device through the De Novo premarket review pathway a regulatory pathway for low- to moderate-risk devices of a new type. This includes activities for labelling control incoming control of materials including labelling and design documentation to ensure labels have adequate space on the device and packaging. Food and Drug Administration FDA develops and administers regulations under authority granted by laws passed by Congress that apply to food drugs cosmetics biologics radiation-emitting electronic products and medical devices.
Labeling 21 CFR Part 801. An Interlaboratory Comparison of Analytical Methods for Ethylene Oxide PB 86-. And regulations administered by other Federal agencies.
Draft Guidance for Industry and Food and. Along with this authorization the. This is a requirement for all finished device manufacturers as well as importers of medical devices.
1 The label of every medical device shall bear a unique device identifier UDI that meets the requirements of this subpart and part 830 of this chapter. Manufacturers must also list their medical devices with the FDA at the time they plan to. Subpart B - Labeling Requirements for Unique Device Identification 80120 - Label to bear a unique device identifier.
The FDA requirements for labelling of medical devices are embedded with Quality System Regulation requirements mentioned in 21 CFR Part 820. The Food and Drug Administration has many labeling-related requirements to help assure that devices. Registration and listing 21 CFR Part 807.
Establishment registration Medical Device Listing.

The Definitive Guide To Ifu For Medical Devices

Technical Documentation Technical File Precondition For Approvals
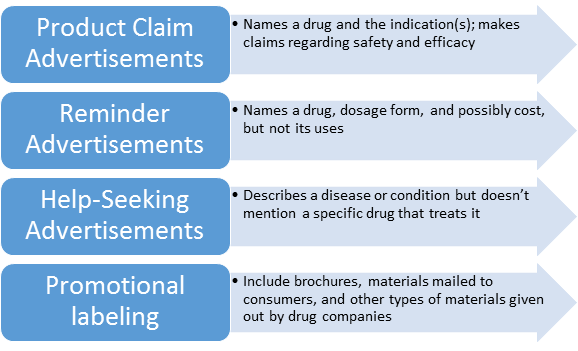
Understanding The Fda Regulations Governing Advertising And Promotion Of Drugs And Medical Devices

The Regulation Of Wearable Medical Devices Trends In Biotechnology