Selling Medical Devices Without Fda Approval
You need approval from the FDA Food and Drug Administration to import and sell medical devices in the United States of America. Food and Drug Administration FDA approval and conspiring to defraud the United States by concealing the illegal sales activity.
Https Www Emergogroup Com Sites Default Files Emergo Fda 510k Data Analysis 2017 Pdf
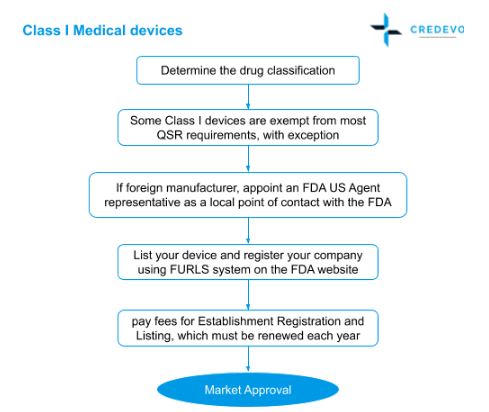
Class I devices and some Class II devices that do not require FDA approval and premarket notification are called 510exempt.

Selling medical devices without fda approval. The steps below provide a brief overview of how the PMA and the FDA 510k process work. Most Class II devices require premarket notification or 510k and most Class III devices require premarket approval or PMA. Also register your establishment with the FDA.
All Class I exempt devices. You wont receive a certificate from the FDA yet this letter will be accessible on the FDA database as confirmation to your clients that your product is cleared for sale. Therefore all concerned establishments are strongly reminded that online selling of medical devices and supplies without the corresponding authorizations issued by the FDA ie.
For Class III devices a Pre-Market PMA submission is needed. When unapproved devices are used in medical procedures it presents a public health and safety risk and federal health insurance programs should not foot the bill. Exporting and selling medical devices into the United States of America If you want to sell medical devices in America you need to obtain FDA approval for the device.
In 2015 FDA approved 98 of PMAs accepted for review and 85 of 510ks accepted for review were determined to be substantially equivalent. Catheters and wheelchairs are examples of Class II devices that require FDA clearance prior to marketing. Prepare the marketing application by providing performance data or clinical data if applicable.
VSI and its chief executive officer Howard Root with selling medical devices without US. An indictment was filed today charging Vascular Solutions Inc. To gain approval they must present evidence that the.
But even if those dont require mobile medical application approval by the FDA they still need to protect any data stipulated by HIPAA regulations. If the product labeling makes disease or therapeutic claimsessentially those that claim a product will diagnose cure mitigate treat or prevent diseasethen FDA will likely regulate the product either as a drug which must be FDA approved and requires prior proof of safety and efficacy before selling the product in interstate commerce or biologic which also requires FDA. Products that have been classified by the FDA as medical devices that require FDA clearance or approval that have not been cleared or approved by the FDA for over-the-counter use such as.
Class I devices such as dental floss and bandages are subject to the least regulation. A device that lacks FDA marketing clearance is considered investigational and therefore the company cannot promote advertise or accept orders for it. The FDA considers such activities to be commercialization of an unapproved device The FDA.
Defects in medical devices such as artificial hips and pacemakers have caused severe patient injuries and deaths. Most class I devices are exempt. Devices that are 510 k-exempt can be marketed without FDA clearance.
See examples of cancer tests below. FDA Advisory No2021-0761 Public Health Warning Against the Purchase and Use of the Unnotified Medical Device Product UMBILICAL CORD CLAMP By Administrator2 April 23 2021 The Food and Drug Administration FDA warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the. License to Operate Certificate of Medical Device Notification or Certificate of Medical Device RegistrationCertificate of Product Registration is strictly prohibited.
Basically when the FDA sends you this letter they are not accepting your device they are stating that your device is significantly comparable to the predicate devices chosen in your 510k and which has just been cleared for sale by the FDA and that you are now presently cleared to sell your device. Before a medical device can be legally sold in the US the person or company that wants to sell the device must seek approval from the FDA. Most Class I devices can be self-registered but most Class II devices require a 510k submission.
Class III are for high risk devices that are considered life supporting. Reports published in 2009 through. Approval depends on the risk classification of the device.
Problems related to medical devices can have serious consequences for consumers. The Gray Area FDA Enforcement Discretion. The time period from FDA submission to clearance or approval can feel like an eternity.
Applications with overlapping functionality that may fit the definition of being a medical device. Most PMAs require clinical data in which case a clinical trial would be conducted in accordance with the Investigational Device Exemption IDE regulations. Medical devices that do not have the required FDA approval or clearance cannot be bought and sold for use on patients.
All companies planning to sell a medical device or IVD in the United States need to register their product with the US FDA. As weve said. 60 Zeilen The products in each list contain information about what medical uses the device is.
Call this the maybe category. But what can companies do to get hospitals and providers excited about their technology before launch.

Market Access For Medical Software In The United States Vde Medical Devices And Software
Medical Device Market Approval Process In The United States Credevo Articles
The State Of Artificial Intelligence Based Fda Approved Medical Devices And Algorithms An Online Database Npj Digital Medicine

Market Access For Medical Software In The United States Vde Medical Devices And Software