Fda Medical Device Recalls 2020
Is a vertebral body replaceme. These products are on the list because there is a reasonable chance that they could cause serious health.

Fda Advisory No 2020 746 Product Recall Of Specific Batches Of Ranitidine As Hydrochloride 150 Mg Film Coated Tablet Peptica Food And Drug Administration
Read the Urgent Medical Device Recall letter.

Fda medical device recalls 2020. Z-0067-2020 - BD FACSLyric Flow Cytometer 2-Laser 6 Color Instrument REF. Who May be Affected Health care providers using the affected Alaris Pump System. 1 02072020 Medtronic Inc.
Medtronic Recalls Valiant Navion. Here is what we know about the recall. On February 11 2020 Cordis Corporation sent an Urgent Medical Device Recall letter to all affected customers and provided the following instructions.
The FDA posts summaries of information about the most serious medical device recalls. Medtronic Inc Cardiac Rhythm and Heart Failure CRHF Z-2506-2020 - Patient Connector Model Number 24967. 495384 - Product Usage.
Z-2101-2020 - Kangaroo Joey Safety Screw Spike Set Anti-free Flow Product Code 765559. Is a vertebral body. Z-0958-2020 - Medtronic MiniMed 640G Insulin Infusion Pump Ref Model s MMT-1511 MMT-1711 MMT-1512 MMT-172 MMT-1752 Rx Only UDI.
Abbott recalled 13891 of its coronary dilation catheters used to open clogged blood vessels. 9 Zeilen 2021 Medical Device Recalls. Z-0066-2020 - BD FACSLyric Flow Cytometer 2-Laser 4 Color Instrument REF.
Z-0548-2020 - Boston Scientific Advanix Pancreatic Stent Straight Leading Barb 10F x 3cm UPN. Model 102956 and OUS Model 201-10002 a component of the. In Medical Device Recall Posted November 4 2020 The US.
11052020 Synthes USA Products LLC SYNMESH 22MM X 28MM 88MM HEIGHT TI Product Number. Devicemakers Abbott ResMed and Teleflex all announced high-risk medical device recalls on Feb. A Class I medical device recall is the most serious meaning that use of the medical device could cause serious injuries or death.
Food and Drug Administration FDA is categorizing Medtronics septostomy catheter recall as Class I. Z-2100-2020 - Kangaroo Joey Safety Screw Spike with Flush Bag Anti-free Flow1000 mL Product Code 765100. Z-2505-2020 - CareLink SmartSync Device Manager Model Number 24970A.
Who May be Affected Health care providers using the device during the treatment. Z-0221-2020 - CentriMag Acute Circulatory Support System Motor US. 23 2020 Jacob Bell BioPharma Dive FDAs list of medical device recalls in 2020 contains 32 separate entries with infusion pumps appearing most frequently followed by catheter issues related to detachment or separation of parts of the device.
1 02072020 Medtronic Inc. 495379 - Product Usage. Please note this recall is separate from the BD Alaris Pump Module 8100 keypad recall dated August 4 2020.
Z-0955-2020 - Medtronic MiniMed 630G System with SmartGuard RefModel MMT-1715. Z-0549-2020 - Boston Advanix Pancreatic Stent Straight Leading Barb 10F x 4cm UPN. Becton Dickinson and Company BD Biosciences.
662875 when using BD Trucount Tubes. The FDA has received 50 Medical Device Reports with 10 injuries and 1 death from November 1 2019 to March 1 2020. 3 11052020 Synthes USA Products LLC END RING 10MM DIA0 DEG TI Product Number.
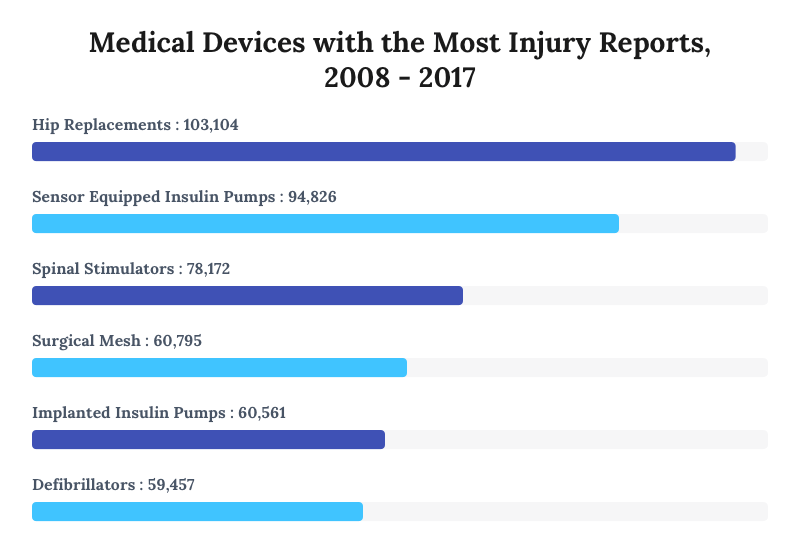
Defective Drugs Medical Devices Injuries Risks And Recalls

Understanding Fda Drug Recall Procedures Hemophilia Federation Of America

Fda Drug Products Recall Enforcement Reports Issued 2012 2019 Statista
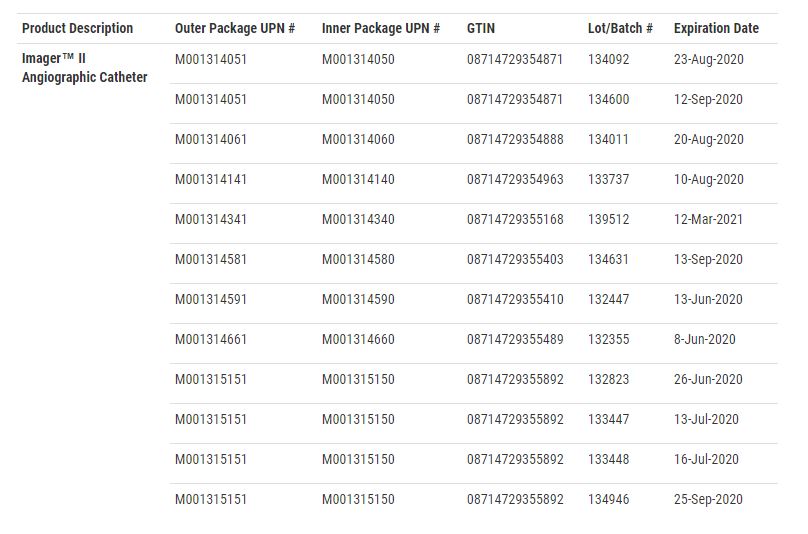
Boston Scientific Medical Device Recall Upgraded To Class I By Fda Drug And Device Watch