Fda Medical Device Component Definition
Manufacturers of human cells tissues and cellular and tissue-based products HCTPs as defined in 12713d of this chapter that are medical devices subject to premarket review or notification or exempt from notification under an application submitted under the device provisions of the act or under a biological product license application under section 351 of the Public Health Service Act are subject. The firm must document procedures that describe what training.
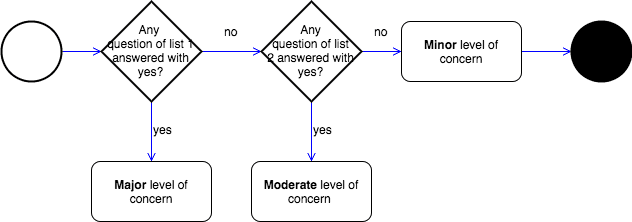
Safety Classes Versus Level Of Concern
An accessory is a finished device that is intended to.

Fda medical device component definition. FDA Updates Definition of Custom Medical Devices 30 October 2016 admin Earlier this month the US FDA updated its definition of a custom medical to include new statutory requirements for custom devices under the Federal Food Drug and Cosmetic Act the FDC Act as amended by the Food and Drug Administration Safety and Innovation Act FDASIA. Accessories might be marketed individually for use with a specific. FDA developed this document to provide guidance to industry and FDA staff about the regulation of accessories to medical devices.
4 Drug product means a finished dosage form for example tablet capsule solution etc that contains an active drug ingredient generally but not necessarily in association with inactive ingredients. 26 Zeilen Medical Device Databases. Staples are sold at office supply stores and FDA doesnt consider them to be medical devices.
December 1997 Previous Page Table Of Contents 3. J Importer means any person who imports a device into the United States and who furthers the marketing of a device from the original place of manufacture to the person who makes final delivery or sale to the ultimate user but who does not repackage or otherwise change the container wrapper or labeling of the device or device package. An exercise component is a device that is used in conjunction with other forms of exercise and that is intended for medical purposes such as to redevelope muscles or restore.
Your device is not a staple. In contrast FDA clarifies that an accessory is a separate finished device intended to support supplement andor augment the performance of at least one parent device. An instrument apparatus implement machine contrivance implant in vitro.
Under 21 CFR 806 Medical Devices. To ensure proper safety and effectiveness. You are confusing a contraption with a medical device.
3 Component means any ingredient intended for use in the manufacture of a drug product including those that may not appear in such drug product. Personnel - 21 CFR 82025. If it is a medical device it is also not a component.
The FDA oversees their safety and effectiveness just like contact lenses that correct your vision. A component in 21CFR 8203 is defined as any raw material substance piece part software firmware labeling or assembly which is intended to be included as part of the finished packaged and labeled device. FDC Act defines a device as.
Section 201h of the Food Drug Cosmetic Act. Reports of Corrections and Removals manufacturers and importers are required to make a report to FDA of any correction or removal of a medical device. Definition of a Medical Device.
A A medicated feed in addition to providing nutrients is a vehicle for the administration of a drug or drugs to animals. Guide to Inspections of Medical Device Manufacturers. When packaged in whole with the stethoscope these parts would be considered medical device components that comprise a finished medical device.
Decorative contact lenses are considered medical devices. 1 medical device means any instrument apparatus appliance software implant reagent material or other article intended by the manufacturer to be used alone or in combination for human beings for one or more of the following specific medical purposes. It is considered a finished devicemeaning it is ready for use or capable of functioning.
Fluoroscopic air kerma display device means a device subsystem or component that provides the display of AKR and cumulative air kerma required. If you repackage or otherwise change the container. This database contains key device identification.
Medical Device Accessory Classification Request Granting Decisions What is a medical device accessory. This guidance is intended to describe FDAs policy concerning the.

Software As Medical Device Samd Classification And Definitions
From Impd To Ind Same But Different Biopharma Excellence

Risk Management For Medical Devices As Defined By Iso 14971 Risk Management Strategies Risk Management Management
Https Www Fda Gov Media 131268 Download