Fda Guidance Shelf Life Of Medical Devices
Establishing Shelf Life of Medical Devices Introduction The FDA defines shelf life as the term or period during which a device remains suitable for its intended use. Shelf Life 21 CFR 8033 Shelf life is the maximum time a device will remain functional from the date of manufacture until it is used in patient care.

Fda S Unique Device Identifier Successful Implementation
So given that I can show the FDA Reviewer that the product will function for for 10 years 5 years and obviously when new safely and effectively.
Fda guidance shelf life of medical devices. This article discusses what this means in practice for medical device manufacturers. Register and download FDA guidance. And read more below.
The useful life of a device is the time a device is in use or in distribution for use. The guideline for stability studies for pharmaceutical products are ICH guideline Q series and there is an FDA guidance for shelf life of medical devices. TUrNEr MEDicAl ENGiNEEriNG TEchNoloGiES lTD.
Requirements and specific criteria necessary to design studies done on product that is aged using both real-time and accelerated aging conditions parameters for establishing stability of the device and device packaging. CFR requirements FDA ISO ASTM European guidelinesstandards that are applicable to the requirement for determination of shelf-life and stability for medical devices. FDA issued final guidance Friday that clarifies the timeline a medical device maker should follow when submitting a premarket approval application PMA supplement following plans to change a manufacturing site.
For example a record may be retired if the person maintaining. In the EU MDR it is stated that the stability study including shelf-life is to be compulsorily established for all medical devices under product verification and validation and it should be part of the Technical documentation. Center for Devices and Radiological Health.
Inform readers of the Food and Drug Administration FDA regulations and policies relating to shelf life of medical devices. Since the device relies on a pneumatic pump to deliver pressure to operate the device thus a combination device. FDA Guidance Shelf Life of Medical Devices April 1991 The duration and conditions over which required performance is achieved.
Fitness for use can be impacted by both maintaining sterility of the package and the performance characteristics of the device. Shelf life is the term or period during which a commodity remains suitable for the intended use. Identifying Shelf Life for Non-Sterile Devices In the case of a sterile device recognizing shelf life is easy since the packaging has a pull date.
The European Medical Device Directive MDD requires all sterile medical devices to have an expiration date. Discuss the various parameters that determine the length of time a particular device will remain within acceptable specifications. Und wie der Titel schon sagt beschäftigt sich der das Dokument nur mit OTS-Software die Teil eines Medizingerätes ist.
For certain devices susceptible to degradation that are intended to treat life-threatening conditions eg pacemakers the failure rate should approach zero within the labeled shelf life. By stating there is no shelf life of the device we are claiming it to be theoretically infinite. Inform readers of the Food and Drug Administration FDA regulations and policies relating to shelf life of medical devices.
There are many naturally occurring. CDRH-Guidancefdahhsgov to receive a copy of the guidance. Please use the document number 1300006 to identify the guidance you are requesting.
Das verführt leicht dazu anzunehmen dass die Begriffe im Wesentlichen identisch sind. Center for Devices and Radiological Health The purpose of this document is to. The FDA provides guidance regarding medical device shelf life determination and advises manufacturers to consider several parameters including chemical and physical.
Outline the different activities that can be undertaken to establish the shelf life of a device. The purpose of this document is to. The purpose of this document is to.
Das Guidance-Dokument in dem man die Definition findet heißt Guidance on Off-The-Shelf Software Use in Medical devices. FDA Guidance The United States Food and Drug Administration provides guidance on physi-cal tests to employ to demon-strate that packaging main-tains the sterility of products throughout their shelf life. Inform readers of the Food and Drug Administration FDA regulations and policies relating to shelf life of medical.
This guidance provides FDAs current thinking regarding documentation that should be provided in premarket submissions for medical devices using Off-The-Shelf software. The guidance also explains what FDA considers to be a manufacturing site change. Some non-sterile devices may also have a shelf life although even a 1991 FDA Guidance Document on shelf life is not clear if that definition only applies before the device is first used.
Said pump has alarms visual and audible if the. FDA has developed this guidance document to assist industry in preparing Premarket Applications PMAs Humanitarian Device Exceptions HDEs.
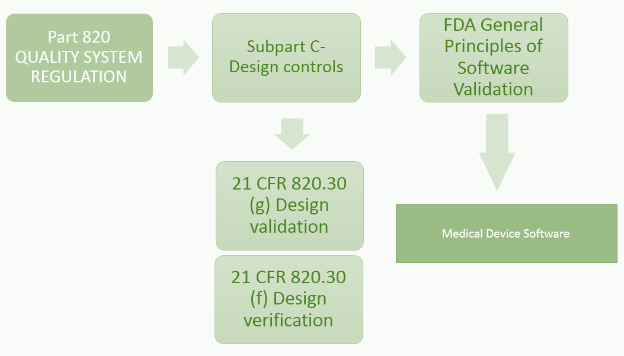
Medical Device Software Challenges By Understanding Fda S Software Regulation Strategy
Fda Finalizes New System To Identify Medical Devices

Medical Device Software Iec 62304

The Definitive Guide To Ifu For Medical Devices