Fda Regulations For Medical Devices Ppt
Changes to existing designs should be made in accordance with design control requirement even if the original design was not subject to these requirements. Rules for IVD medical devices are set out in Schedule 2A.

Us Fda Medical Device Or Equipment
This online reference for CFR Title 21 is updated once a year.

Fda regulations for medical devices ppt. Medical Device Reporting----the Final Rulethe Final Rule 1. Intended use of the device. Medical Device Reporting MDR Establishment Registration - 21 CFR Part 807 Manufacturers both domestic and foreign and initial distributors importers of medical devices must register their.
The resilient packaging must also meet rigorous labeling standards which let the FDA trace devices in use. When fully implemented the. The FDA has pre-determined approximately 1700 different generic types of medical devices.
The US has 310 million people and the. FDA is establishing a unique device identification system to adequately identify medical devices through their distribution and use. A medical device is The Section 201h of the Food Drug and Cosmetic Act defines a medical device as any healthcare product that does not achieve its principal intended purposes by chemical action or by being metabolized.
Each of these device types is allocated to 16 different medical panels. Manufacturers and initial distributors of medical devices must register their establishments with the FDA. The medical device design control section of the FDA Quality System Regulation applies to the design of products and processes and changes to existing designs and processes.
Introduction to US FDA Medical Device Regulatory Process. All establishment registrations must be submitted electronically unless a waiver has been granted by the FDA. Classification The classification is risk based and determined by a set of rules in Schedule 2 Part 1 of the Therapeutic Goods Medical Device Regulations 2002.
All registration information must. PRE-MARKET ASSESSMENT 9 SECTION 1. As simple as a tongue depressor or a thermometer.
Medical device requirements are basically the same in most countries but are implemented in different ways 10. Classification Determined by the rules in Schedule 2 and 2A Part 1 of the Therapeutic Goods Medical Device Regulations 2002 14. For the most up-to-date version of CFR Title 21 go to the Electronic Code of Federal Regulations eCFR.
Premarket Approval PMA 21 CFR Part 814. FDA Medical Device Rules Robert F. Domestic distributors no longer have to submit MDR reports but they must continue to maintain records of 23 Copyright 1999 -2009 Glisland Inc.
This database includes a codification of the general and permanent rules published in the Federal Register by the Executive departments and agencies of the Federal Government. Medical device manufacturers importers and distributors are no longer required to submit and annual certification statement Form FDA 3381. FDA Responsibility for Medical Devices FDA regulates medical devices including.
Act 1938 Radiation Health and Safety Act 1968 Medical Device. FDA Regulation of Medical Devices Congressional Research Service 1 Introduction Medical device regulation is complex in part because of the wide variety of items that are categorized as medical devices. They may be simple tools used during medical examinations.
Lets look at the basic regulations that US-based manufacturers and distributors must comply with and understand them one by one. Each generic device is then assigned to one of the Classes ie. The European Medicines Agency EMEA regulations.
DRAFT Guidance Document Medical Devices Regulatory System V119012005 GUIDANCE DOCUMENT MEDICAL DEVICES REGULATORY SYSTEM Table of Contents PART 1. Premarket Notification 510k 21 CFR Part 807 Subpart E. Class I Class II Class III based on the potential patient risk associated with the device.
In short the medical device development is not limited to the devices themselves but. Title 21 of the CFR is reserved for. Types of FDA Regulations for Medical Devices.
FDAs Center for Devices. USA The FDA regulates food drugs medical devices biologics cosmetics and radiation emitting products in the USA. INTRODUCTION 9 11 Principles and Main Features of A Regulatory Framework of Medical.
Risk classification is based on. FDA Medical Device Rules. PowerPoint PPT presentation.
Device The FDA requires all medical device manufacturers to register their facilities and list their devices with the agency. Pre 1976 Devices regulated under drug authorities May 28 1976 - Medical Device Amendments PL 94-295 Safe Medical Devices Act SMDA of 1990 FDA. Simple items like tongue depressors and bedpans complex technologies such as heart pacemakers dental devices surgical implants and prosthetics devices used to diagnosis disease or.
The United States is the number one medical device market in the world accounting for more than 40 of all healthcare spending worldwide. Establishment Registration.
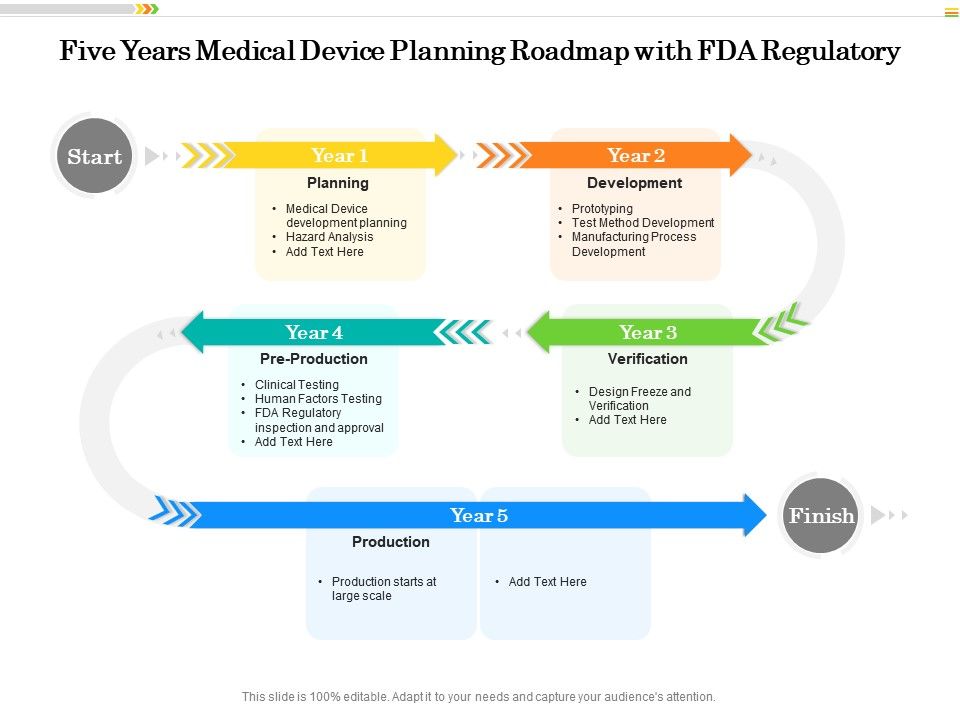
Five Years Medical Device Planning Roadmap With Fda Regulatory Presentation Graphics Presentation Powerpoint Example Slide Templates
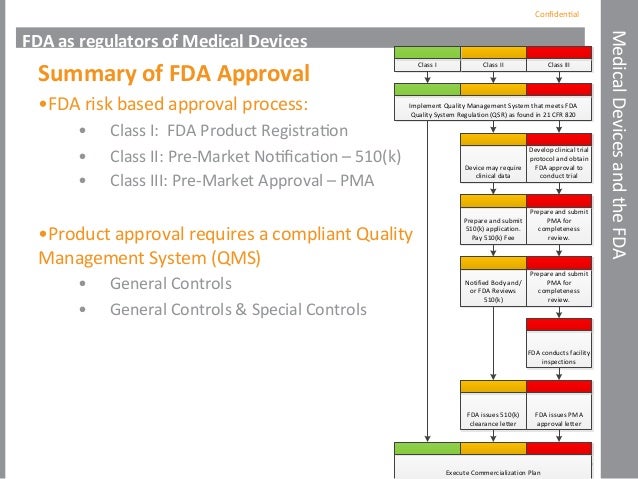
Fda Regulations And Medical Device Pathways To Market
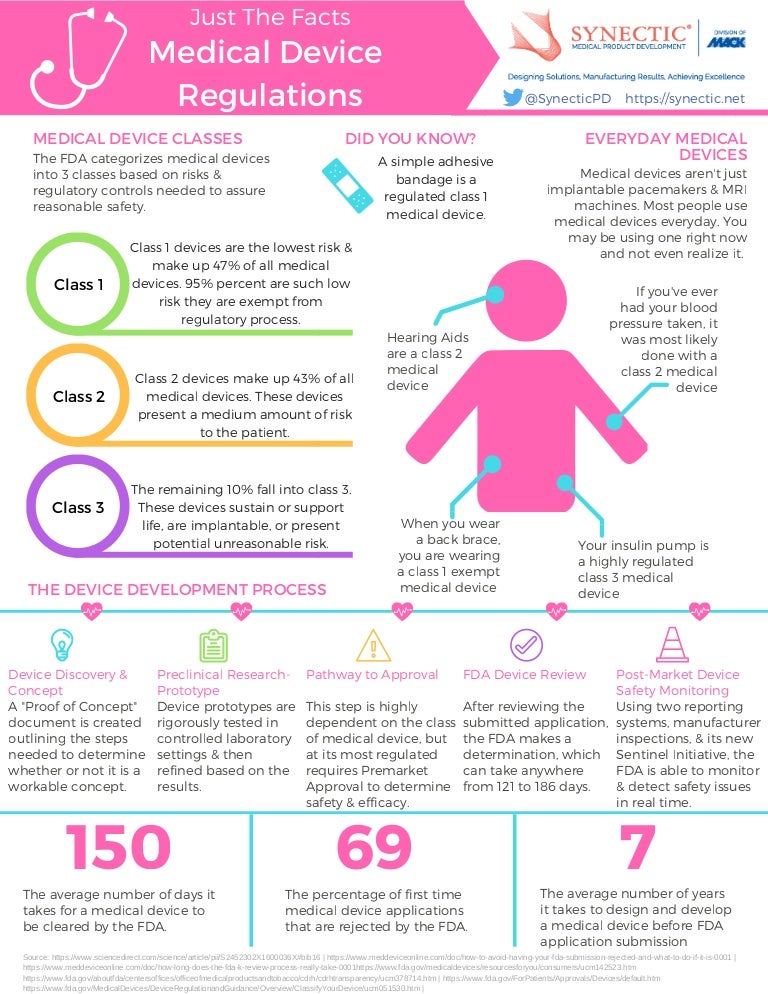
Medical Device Fda Regulations And Classifications Infographic

Fda Regulation For Medical Devices