Fda Class 1 Exempt Medical Device
Class 1 Medical Devices have the lowest risk perceived. Where appropriate this information should take the form of symbols.
FDA is also amending the codified language for the list of class II devices to reflect this final determination.
Fda class 1 exempt medical device. What may not be evident or often discussed in common conversation is that many Class I and some Class II medical devices are 510k exempt. However you are required to register your establishment with the FDA by registering your company and listing the medical. Many Class 1 medical devices have been exempt from the premarket application process.
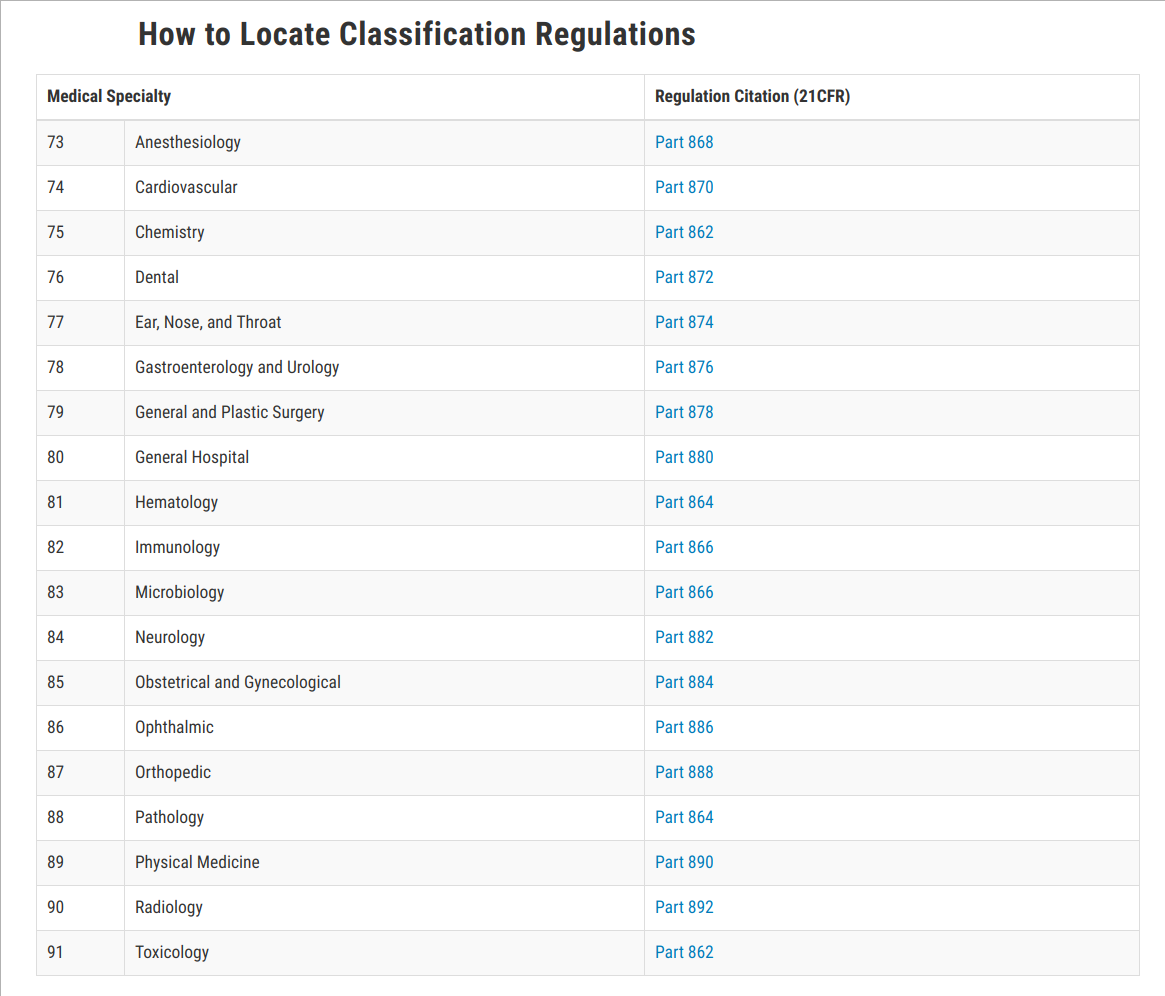
A Class 1 device is the least likely to cause harm. Class I Devices FDA has exempted almost all class I devices. If your device falls into a generic category of exempted Class 1 devices as defined in 21 CFR Parts 862-892 a Premarket Notification 510k clearance may not be required unless you are introducing a new and unique technology or unless you modifychangeexceed the intended use.
Class 1 Medical Device Class 1 medical device can be self-declared for CE compliance as per the MDR. December 30 2019 By Nancy Crotti The FDA today published a list of Class I and Class II medical devices that it now considers exempt from premarket notification in accordance with the 21st. Elastic bandages examination gloves etc Many Class I devices are exempt from PMN andor QS regulation requirements Available predicate No available predicate Low or moderate risk High risk de novo device Not de novo device Pre-market notification.
These exemptions took effect January 15 2021. By way of exception no such instructions for use are needed for devices in Class I or IIa if they can be used safely without any such instructions. Anyone can determine whether a device is exempt from 510 k or GMP requirements by searching the FDAs Product Classification database.
Overview of FDA regulatory pathways for medical devices. If a manufacturers device falls into a generic category of exempted class I devices as defined in 21 CFR Parts 862-892 a premarket notification application and FDA. Self-declaration means neither the Notified Body certification is required nor any other kind of approvals from any certification bodies.
By way of example a toothbrush is a Class 1 exempt device. The exemption will decrease regulatory burdens on the medical device industry and will eliminate private costs and expenditures required to comply with certain Federal regulations. If your device is classified as.
According to a recent FDA notice the seven Class I device types permanently exempt from 510 k requirements include surgical and examination gloves. What does 510k exempt mean. Class 2 devices require a 510 k application to be submitted and approved prior to.
Device New device Class I Low risk. Instructions for use must be included in the packaging for every device. This exemption from 510k subject to certain limitations is immediately in effect for the list of class II devices.
FDA Medical Device Exemptions and Registration Procedures Feb 21 2020 The Food and Drug Administration FDA the US agency responsible for medical device regulation has published a list of Class I and Class II medical devices exempt from the obligatory premarket notification requirement. When a 510k submission is required it means that the FDA is requesting notification along with evidence that the medical device intended to be marketed is safe and effective prior to a company commercializing its product. The class to which your device is assigned determines among other things the type of premarketing submissionapplication required for FDA clearance to market.
The 510 k notifies the FDA that you plan to market a product SE to others already in the market. 510 k Exemptions Most Class I and some Class II devices. Andrew Kyle If the device is exempt you must still maintain a DHF and it must document the predicated Class 1 devices You may have the DHF reviewed during routine GMP visits and it must maintain the same integrity as a non- 510 k product.
Learn how medical device markets are reacting to COVID-19 VISIT THE COVID-19 RESOURCE CENTER Additional Class II device exemptions targeted. Under 510 1 1 a class I device is exempt from the premarket notification requirements under section 510 k of the FDC Act unless the device is intended for a use which is of substantial importance in preventing impairment of human health or it presents a. Only devices annotated by are also exempt from GMP except for general recordkeeping requirements and compliant files.

Dermaneedling Instruments Are Not Fda Approved Fda Dermapen Medical Advice
Eu Mdr Requirements For Class I Medical Device Manufacturers
Eu Mdr Requirements For Class I Medical Device Manufacturers
Does An Fda Class 1 Medical Device List Exist
