Fda Vs Eu Medical Device Regulation
However after recent high-profile device failures political pressure in both the United. The adoption in April 2017 of Regulation EU 2017745 on Medical Devices MDR and Regulation EU 2017746 on In-Vitro Diagnostic Devices IVDR changed the European legal framework for medical devices introducing new responsibilities for EMA and for national competent authorities.
How The Eu Medical Device Regulation Changes Ce Marking Compliance
FDA approval means that the device is approved for use in all parts of the world while the CE mark has restrictions sometimes even within the EU.

Fda vs eu medical device regulation. FDA regulates false eyelashes and artificial nails for example as cosmetics. Differences in Regulatory Framework. The Consumer Product Safety Commission has jurisdiction over many non-medical devices that people use to affect their.
EUs Medical Device Regulation By. Guidelines are now adopting similar techniques to ensure uniform standards applied to devices across the board. The FDA approval process mandates that a device be proved efficacious compared with a control or be substantially equivalent to a predicate device whereas the European Union approval process mandates that the device perform its intended function.
The EU justifies this classification based on the. Both the EU and FDA classify a pacemaker as a class III device. Stringent peer-reviewed safety data have not been reported.
EU vs US US GMP requirements detailed in Title 21 CFR Code of Federal Regulations has legal binding force EU GMP requirements Regulations Directives Guides eg. It is composed of an electrical circuit which stays within the body after surgery. Thus whereas the FDA has the advantages of centralization and common rules the European Union regulates medical drug and device approvals through a network of centralized and decentralized agencies throughout its member states.
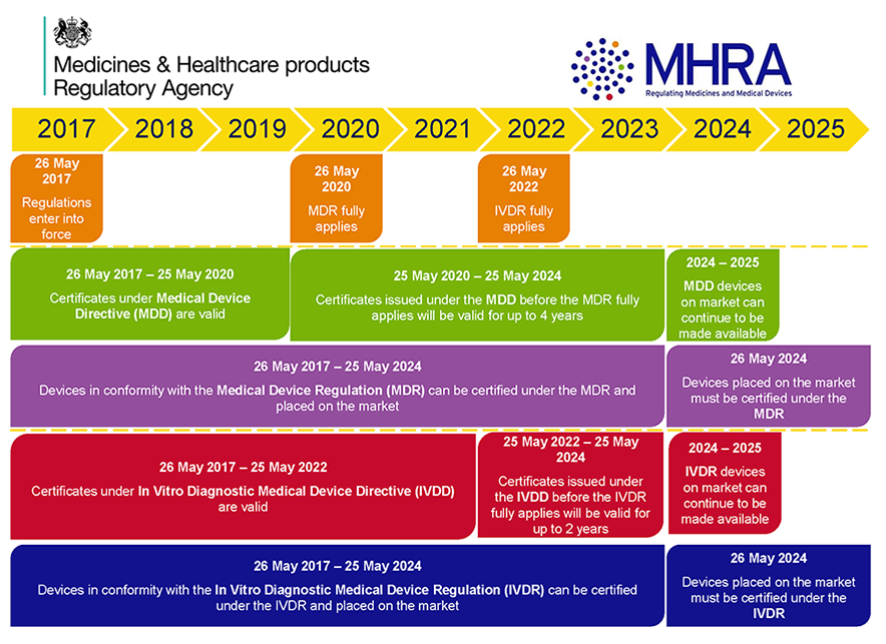
Both Regulations entered into force in May 2017 and have a staggered transitional period. Es wurde eine dreijährige Übergangsfrist vereinbart das Gültigkeitsdatum wurde im Jahr 2020 aufgrund der Corona Pandemie um ein Jahr verlängertDie neue MDR ist also ab dem 26. Die Verordnung EU 2017745 über Medizinprodukte ist am 25.
Hills and Dan Kagan In follow-up to our colleagues recent post about the newly implemented Medical Device Regulation MDR in the European Union this post will discuss some of the similarities between FDAs Clinical Decision Support CDS Software Draft Guidance together the CDS Draft Guidance. Die Medizinprodukteverordnung MDR Medical Device Regulation zusammen mit der IVDR In vitro Diagnostic Regulation ist seit dem 25Mai 2017 gültig. The initial intention was that all classes of devices will comply by Sep 2018 however later on the FDA published its decision NOT to enforce the class Iunclassified device compliance dates for additional two years.
The new EU Medical Device Regulation MDR published in 2017 also set a requirement for UDI. EU-Verordnung für Medizinprodukte PDF 16 MB. A cardiovascular medical device that I love learning about it the pacemaker.
Sie wird auch Medical Device Regulation MDR oder europäische Medizinprodukte-Verordnung genannt. Sie gilt in den Mitgliedstaaten der Europäischen Union unmittelbar und muss daher nicht in nationales Recht umgesetzt werden. Mai 2017 in Kraft getreten.
Regulations have binding legal force in every Member State MS and enter into force on a set date in all the MSs. Gleichwohl werden umfängliche Anpassungen des nationalen. Die Medical Device Regulation MDR Europäische Verordnung für Medizinprodukte trat gemeinsam mit der Verordnung für In-vitro-Diagnostika IVDR am 25Mai 2017 offiziell in Kraft.
Mai 2021 verpflichtend anzuwenden. Die MDR ist nach einer vierjährigen Übergangszeit ab 26. EUs Medical Device Regulation.
As one medical device company founder says of the CE marking there is no guarantee that the device will be widely accepted by physicians or reimbursable by the government in each European country Chi 2012. For example the movement of European guidelines to a stricter review and audit of not only the QMS but. FDA Clinical Decision Support Software vs.
FDA Clinical Decision Support Software vs. Medical device regulatory compliance under both the FDA and the EU MDR is a complex and continual process involving processes that need constant monitoring and maintenance. In follow-up to our colleagues recent post about the newly implemented Medical Device Regulation MDR in the European Union this post will discuss some of the similarities between FDAs Clinical Decision Support CDS Software Draft Guidance together the CDS Draft.
A pacemaker is considered in the EU as an Active Implant Medical Device.
Mhra S Guide To The New Eu Medical Devices Regulations Bioslice Blog

Differences Of The Approval Of Medical Devices In The Usa And Europa Eu Download Scientific Diagram

Device Regulations The New Medical Device Regulation The Applicability Of Article 117 To Medicinal Products